Manufacturing
Latest News
Latest Videos

More News

Collecting and curating R&D data are increasingly crucial tasks for achieving the full benefits of advanced analytics.

The design of the center prioritizes integration of the entire supply chain, with the end goals of accelerating product development and autonomous production capacity in alignment with EU priorities.

Ahmed Youssef, senior manager, USP Process Development at Ascend, provides insight on tech transfer when developing and manufacturing emerging therapies and new modalities.

Evonik won the CPHI Excellence in Pharma Award in the “Sustainability” category in recognition of its plant-based squalene, PhytoSquene, used in parenteral drug delivery applications.

SK pharmteco will be the preferred manufacturing partner for AVG-101, AaviGen’s lead gene therapy product.
Pharmaceutical packaging must advance to adapt to new, complex modalities.
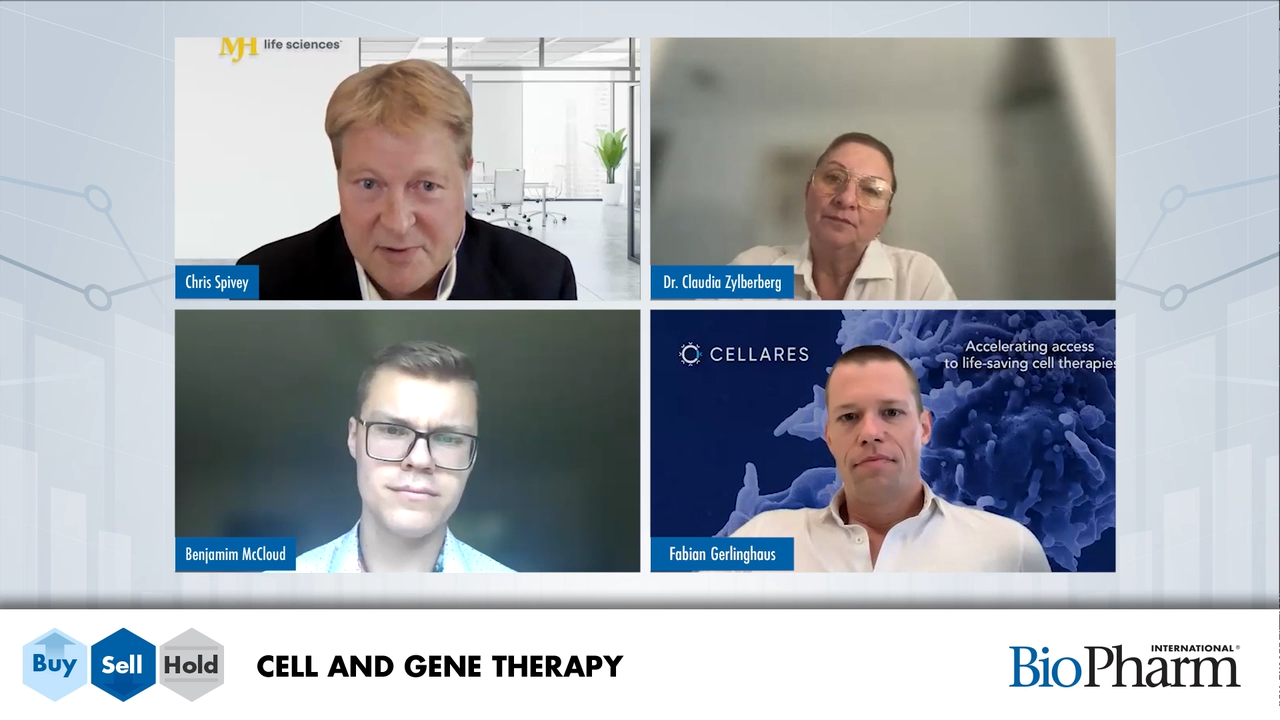
Cell and gene therapy experts Fabian Gerlinghaus, Dr. Claudia Zylberberg, and Benjamin McLeod weigh in on hot topics in CGT.

Advancing Injectable Drug Quality: Innovations, Compliance, and Sustainability in the Healthcare Industry
Webinar Date/Time: Tue, Nov 5, 2024 9:00 AM EST

Various microorganisms, including molds and bacterial spores, were tested on stainless-steel coupons and demonstrated 2–6 log reductions with one-minute wet-contact times.

Enzene Biosciences CEO Himanshu Gadgil talked about fully connected continuous manufacturing's role in providing equity for biotech companies that may not normally be able to afford such services.
Nicolas Camper, senior director—Bioconjugation Chemistry, Abzena, discussed new advancements in ADC technologies, including the role of complex chemistries.

Through the global partnership, Colorcon will exclusively represent LOTTE’s AnyCoat Hypromellose products.

Pharmaceutical Technology Europe spoke with Ali Rajabi-Siahboomi, vice president and chief innovation officer at Colorcon, about the evolution of coating design and how it has impacted investment, as well as innovations in packaging for oral solid dosage products.
Pharmaceutical Technology® Europe sat down with Hanns-Christian Mahler from ten23 health to discuss the fill/finish market and sustainability considerations for facilities.

Zaim Gashi, area sales manager of Steriline, discussed the evolution of aseptic processing equipment in line with current regulatory requirements at CPHI Milan.

According to the annual survey, four of the five biggest biologics capacity holders in 2028 will comprise CMOs, which are expected to have 45% of all CMO capacity in Asia.

As a GMP manufacturing method, 3D printing offers benefits for modified release and personalized dosages.

Stacey Treichler, senior director of global marketing and strategy, Purolite, an Ecolab company, spoke about current trends in the biopharma industry and innovations in purification resins at CPHI Milan.
Andrew Lewis, PhD, Chief Scientific Officer, Quotient Sciences, discussed the CMC challenges with GLP-1 and oral peptides during CPHI Milan.

Pharmaceutical Technology Europe chats with Klaus Ullherr, senior product manager at Syntegon, about challenges facing companies when trying to comply with Annex 1 and how automated systems can help.

Webinar Date/Time: Fri, Oct 25, 2024 11:00 AM EDT

Important standards and guidelines for packaging protect and assist the patient.

The nanofiber microcarriers are the first of their kind for manufacturing viral vectors used in gene therapy production, according to Cellevate.

Aaron Cowley, PhD, chief technical officer and co-founder, Captozyme, discussed the advancement in development of microbiome-derived therapeutics at BIO 20204.
In this exclusive Drug Digest video interview, Felicity Thomas, Associate Editorial Director, Pharmaceutical Technology Group, interviews experts about key trends impacting small-molecule APIs and excipients, the importance of supply chain resilience, ways in which advanced manufacturing approaches can prove beneficial, and potential hurdles facing companies seeking to secure their small-molecule API and excipient supply chains.