
Manufacturing
Latest News
Latest Videos

More News

Executives at Avantor talk about the future of the biopharmaceutical industry and the anticipated impact that a wave of next-generation biotherapeutics will bring.
Brian Feth and Jonathan Grinstein go behind the headlines to discuss the impact of a Republican administration on Federal Trade Commission actions, tax relief, and Health and Human Services (HHS) leadership; as well as advancements in CAR-T.

Under an ongoing partnership with Merck, known as MSD outside of the United States and Canada, Ginkgo Bioworks has achieved the first milestone in a project aimed at improving biologics production.

Manufacturers of five autologous or matched allogenic cell therapy products have selected TrakCel's IT platform, OCELLOS, to orchestrate the administration of these therapies, which are approved or expected to be approved in 2024.

Ascend is partnering with EW Healthcare Partners to expand capabilities in the United States.

Evolving industry demands are driving the need for greater flexibility and innovation in processing equipment for pharmaceutical manufacturing.

The companies will work to create climate-friendly pMDIs using Orbia’s low global warming potential propellant.

Pharmaceutical training programs are enhanced by integrating ICH quality risk management considerations.

Synaffix, a Lonza company, has licensed its ADC technology to BigHat Biosciences to be combined with the latter's ML design platform to develop new ADCs

Shifting to automated, closed modular manufacturing systems is growing increasingly crucial for biopharmaceutical production.

Capturing and curating R&D data are crucial to realizing the full value of advanced analytics.

The complexities of tech transfer may be overcome by data-driven approaches, digital tools, and effective communication.

Recent hurricanes in the US close Baxter plant, shining a spotlight on supply chain fragility again.

The ROSS FDA-50 Fixed Tank Dual Shaft Mixer is built to handle a wide range of formulations and viscosities and is ideal for processes that require meticulous control over mixing, temperature, and pressure.

The Mobius ADC Reactor is the first single-use reactor designed specifically for the manufacture of antibody drug conjugates.
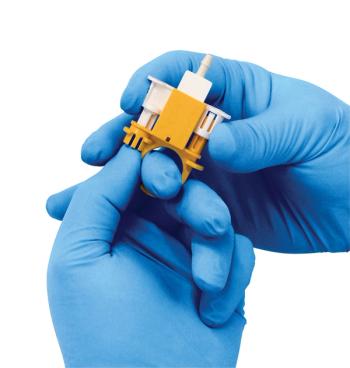
CPC’s MicroCNX ULT Series represents the first aseptic microconnector that can be fitted directly into freeze cassettes for GCT production.

With DKK 600 million (US$87.4 million) in funding from the Novo Nordisk Foundation, the new supercomputer offers the potential to accelerate innovation in drug discovery.

The partnership will use ViaNautis’ proprietary polyNaut technology platform to develop genetic medicines that can precisely target tissue types.

Webinar Date/Time: Fri, Nov 22, 2024 11:00 AM EST

Emerging therapies, patient-centric medicines, and the ever-changing world of bio/pharmaceuticals complicate technology transfer.

POLYOX™ Polyethylene Oxide Essentials: An Expert Guide to Polymer Properties and Drug Formulation
Webinar Date/Time: Tue, Nov 19, 2024 11:00 AM EST
Laks Pernenkil, Brian Feth, and Alex Philippidis go behind the headlines to discuss the impact of recent news, including FDA’s drug shortage list update, Nobel prize winners in microRNA and AI, and a big-potential research win for RNA editing.
In this episode of the Ask the Expert video series, Peter Walters, Fellow of Advanced Therapies at CRB Group, discusses how facilities used for solid dosage manufacturing may be retrofitted into sustainable cell and gene therapy production facilities.
Shawn Li, principal scientist at Merck & Co., known as MSD outside the Unites States and Canada, talked about the importance of properly characterizing HCPs in the biomanufacturing process.

At AAPS PharmSci 360, Karunakar Sukuru, RPh, PhD, the global vice-president-Rx Product Development, Pharma and Consumer Health, at Catalent Pharma Solutions, discussed lipid-based formulations and tackling bioavailability challenges for oral biologics.













