
Drug Development
Latest News


Drug manufacturers need to work closely with excipient suppliers to ensure supply chain safety.
More agile techniques are improving the development of multiparticulate drug-delivery systems.

The authors look at challenges and considerations to continuously improve inspection efficiency.

FDA issued approvals for less than half the number of new drugs in 2016 compared with 2015.

The £14-million research project aims to better understand adverse drug reactions through a variety of modeling approaches.

In a recent deal with the Federal Trade Commission, Endo agreed to refrain from entering into future pay-for-delay agreements for ten years.

Merck will pay a one-time fee of $625 million and additional royalties to BMS and Ono Pharmaceutical to settle the patent infringement case related to Keytruda.

New website allows access to research quantities of commercial-grade ligands and catalysts.


The partnership will focus on providing practical information to clients on the development of biologics and vaccines.

GEA’s ConsiGma continuous tableting line combined with Siemens’ automation and Sipat data management systems enables continuous manufacturing.

Alternatives to expensive palladium catalysts are gaining acceptance for commercial API synthesis.

As of mid-December, less than half the number of new drug approvals were issued by FDA in 2016 compared with 2015.

As pharmaceutical quality metrics evolve, they will need to incorporate more of the principles of operational excellence, says consultant Prabir Basu.
Isothermal titration calorimetry and differential scanning calorimetry are valuable tools that can help accelerate drug development.

AGC adds second biopharma contract manufacturer with acquisition of CMC Biologics.

A pilot project, beginning in 2017, will support the development of biosimilars.

The companies entered a manufacturing agreement for the future commercial production of Lenti-D and LentiGlobin product candidates.

Oxford Genetics received £1.61 million from Innovate UK to explore computational and synthetic biology approaches for optimized mammalian bioproduction.

Saneca Pharma has received a EUR1.5 million grant from the Slovak Ministry of Education, Science, Research, and Sport, which will be used to drive forward new R&D initiatives for API manufacturing.

Previous hesitation by pharma industry to use cocrystals may change with FDA’s new guidance that classifies cocrystals APIs.

Researchers test the efficacy of a new polymer that is an alternative to PEG for drugs used to treat type 2 diabetes.

FDA and BARDA awarded a contract to Continuus Pharmaceuticals to develop an end-to-end continuous manufacturing process for solid-dosage drugs.
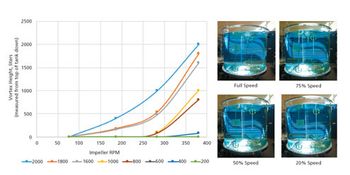
Quantitative and qualitative tools allow better understanding of mixing in a single-use bioprocessing system.


