
Since the passage of the BPCI Act in 2009, manufacturers have been waiting for guidance from FDA on what that approval process will look like.

Since the passage of the BPCI Act in 2009, manufacturers have been waiting for guidance from FDA on what that approval process will look like.

Xevo TQD provides ultimate consistency between analyses; instrument to instrument, lab to lab; both the advanced technology of the ACQUITY UPLC System and the robust universal ion source architecture on the Xevo TQD guarantee flexibility and dependability.

Are biosimilars the next big thing or just the next big bubble?

A path to personalized medicines creates a new paradigm for development and manufacturing.

FDA Approves the Influenza Vaccine Formulation for the 2011-2012 Flu Season.

A bill introduced by Senators Scott Brown (R-MA), Ron Wyden (D-OR), and John McCain (R-AZ) on July 13, 2011, aims to encourage states to reduce Medicaid spending by offering financial incentives to substitute generic drugs for branded ones where possible.

The performance of biotechnology venture capital and investment is lackluster at best.

The solid form of an API plays a crucial role in drug quality, and advancing methods for screening, detection, and characterization is key.

Follow-on versions of complex biologics require extensive expertise in development and regulatory procedures.

A technical leader in solid dosage forms Pfizer CentreSource provides analytical, technical, regulatory, developmental, manufacturing and packaging support that spans the specialized realm of highly potent solid oral dose drug products.

Merck and Hanwha Chemical have formed an exclusive global agreement to develop and a commercialize a biosimilar of Enbrel, a drug to treat moderate to severe plaque psoriasis, psoriatic arthritis, and moderate to severe rheumatoid arthritis.

Effective containment in API and drug-product manufacturing encompasses a variety of process, equipment, and operational issues.
More sophisticated biological expression systems expand the functionality of the traditional systems for protein synthesis.

Academic–industry partnerships are increasingly important in biopharmaceutical innovation.

FDA weighs multiple views regarding the Biologics Price Competition and Innovation Act.

Effective containment in API and drug-product manufacturing encompasses a variety of process, equipment, and operational issues.

Filling active ingredients directly into capsules is probably the quickest option for entering clinical trials. This case study compares manual and automated methods of capsule filling.

FDA is asking for input on the development of a user-fee program for biosimilar and interchangeable biological product applications.
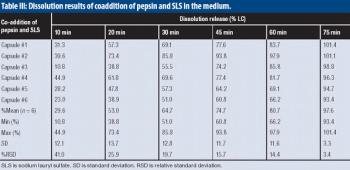
The authors develop a pratical approach to avoid unwanted interactions between pepsin and SLS in dissolution Tier II tests.

Emerging methods could provide alternative ways of producing inhalable drug particles.

Innovator and generic-drug companies need to adapt to compete in the biosimilars market.

The authors question certain aspects of the industry's current regulatory-compliance strategy and suggest that aseptic-process control and evaluation should be revised.

A technical forum moderated by Patricia Van Arnum

The IMS institute has released its report on the use of medicines in the United States during 2010.

We want to fill one of our oxygen-sensitive products inside an isolator system. Can we run an isolator with pure nitrogen and defined humidity?