
Developments in hot-melt extrusion using twin-screw extruders to make solid-dosage drug forms.

Developments in hot-melt extrusion using twin-screw extruders to make solid-dosage drug forms.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the June 2012 edition from EMD Millipore and Meissner Filtration Products.

Novartis, GlaxoSmithKline, and Lonza are among those participating in newly formed consortiums for developing and producing medical countermeasures.

Basilea, Stiefel Enter into Hand-Eczema Pact; Sandoz Recalls Generic Oral Contraceptive Drug; and More.

In addition to the established coating systems (like the BFC and the BFC TriPan), Bohle developed a new coater to face the market needs, the Bohle-Tablet-Coater BTC.

Dr. Reddy's Laboratories and Merck Serono, a division of Merck KGaA, have partnered to codevelop a portfolio of biosimilar compounds in oncology.
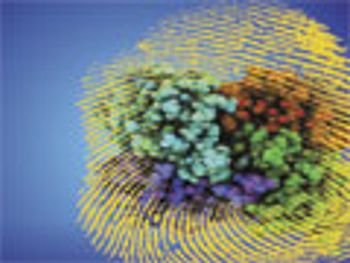
Extensive physicochemical characterization of the innovator product and the proposed biosimilar provides the foundation for demonstrating biosimilarity.

How to avoid invisible and airborne contamination.

Partnerships between industry and academic medical centers are expanding to meet R&D needs.

New coating technologies achieve high uniformity and reduce waste through mixing system advances and pan and airflow configuration.

Does global development have to entail multiple comparability studies?

PDA Revises Technical Report on Sterilized Products

The Parenteral Drug Association has released guidance on the detection and mitigation of 2,4,6-tribromoanisole and 2,4,6-trichloroanisole taints and odors in pharmaceutical and healthcare products.

Advances in process analytical technology and in defining lots for release are key for enabling use of continuous processes in solid-dosage manufacturing.

Coherus BioSciences, Daiichi Sankyo Form Biosimilar Pact; Sandoz Agrees to Acquire Fougera Pharmaceuticals; and More.

AstraZeneca's CEO David Brennan To Retire; PRA, Amgen Reach New Biosimilar Agreement; and More.

A technical forum featuring Tim Freeman of Freeman Technology and Carl Levoguer of Malvern Instruments.

The Obama administration details five strategic imperatives to drive bioscience research as a means of economic growth.

Industry experts working with extended-release injectables discuss challenges and solutions to formulating and manufacturing these complex products.
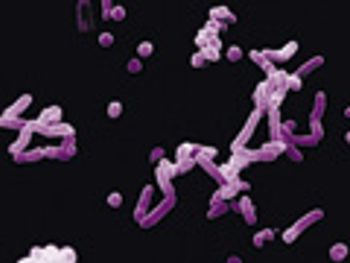
In this technical forum, experts describe different methods of rapid microbial testing and their applications.

Closed-vial technology is an alternative to traditional glass vial filling that reduces the risk of contamination for the patient, simplifies the filling process, and provides easier handling for healthcare providers.

The European Commission is offering a EUR 2-million ($2.64 million) inducement prize to encourage innovation in the area of cold-chain logistics and vaccine stability.

The growing use of high-potency APIs is leading to changes in how the pharmaceutical industry looks at containment.

FDA has released a final guidance describing cGMP for preparing media fills for validation of aseptic preparations for positron emission tomography drugs.

As part of an ongoing collaboration into tabletting science, I Holland and the UK's University of Nottingham have launched a two-year programme to investigate the cause of tabletting sticking.