
Also, Penn Pharma to expand; stem cell research funding ban lifted; Bristol-Myers Squibb made senior appointments; more...


Also, Penn Pharma to expand; stem cell research funding ban lifted; Bristol-Myers Squibb made senior appointments; more...

Also, Schering-Plough's vaccine unit, Nobilon, formed an agreement with the World Health Organization; Ore Pharmaceuticals named president and CEO; more...

Ultra high performance liquid chromatography is advantageous in a contract laboratory because it is faster, more sensitive, and relies on smaller volumes of organic solvents than HPLC.

USP's Stage 2 heparin monograph revisions address identification, potency, and impurities.

Industry has changed, but its basic tenets have not. INTERPHEX's RJ Palermo discusses a 7-step process to keep pharma moving forward.
The authors extend the range of a near-infrared calibration model for tablet assay using production 'seed' spectra and synthetic spectra generated from placebos and 'pure' active pharmaceutical ingredient spectra.
Editors' Picks of Pharmaceutical Science & Technology Innovations

Brief pharmaceutical news items for March 2009.

Contract manufacturers of APIs and intermediates report gains, but express caution.
The author tests the ruggedness of VRL viewing conditions and defines optimal viewing conditions.

An updated book provides essential information for scientists who monitor microbial quality.
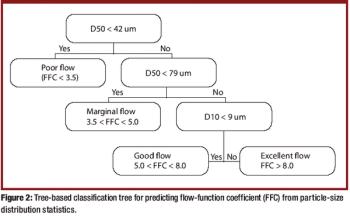
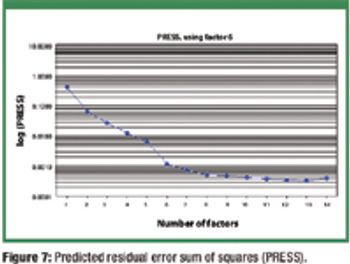
The authors present a simple and material-sparing approach for estimating the powder-flow performance of previously uncharacterized single-component bulk powders when only particle-size distribution data are available.
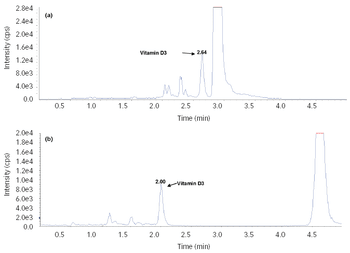
An LC–MS/MS method for the quantitative determination of vitamin D3 in human plasma has been developed and validated with positive atmospheric chemical ionization sources.
PAT guidance has been available from FDA for more than 4 years, but there have been no apparent breakthroughs in large-scale upstream production. Will companies consider using on?line chromatography to change this?

Development & Validation of an Inductively coupled Mass Spectrometric Method-ABC Labs Whitepaper

Also, GPC Biotech AG and Agennix to merge; BASi appoints COO of scientific services; more...

Also, Sandoz received approval for its third biosimilar from the EU, WuXi PharmaTech's CFO Benson Tsang to leave at month's end; more...

To implement QbD and reduce business risks, teams should begin QbD collaboration early during process development.

Congressman John D. Dingell (D-MI) introduced HR 759, known as the Food and Drug Globalization Act of 2009, which would amend the Food, Drug, and Cosmetic Act to address food, drug, and device safety, including registration of producers of drugs and applicable fees, documentation for admissibility of drug imports, country of origin labeling, and the inspection of producers of drugs and active pharmaceutical ingredients (API).

Also, PPD to acquire AbC.R.O.; Bilcare Global Clinical Supplies named Tony Moult general manager of Bilcare GCS Europe; more...

The United States Pharmacopeial (USP) Convention is pursuing greater collaboration with the US Department of Health and Human Services (HHS), and specifically, the US Food and Drug Administration.

Also, recalls for two KV Pharmaceutical subsidiaries; Human Genome Sciences delivers anthrax drug to US Strategic National Stockpile; Akorn president and CEO leaves the company; more...

The Federal Trade Commission has filed a complaint in federal district court challenging agreements in which Solvay Pharmaceuticals (Marietta, GA) paid generic drug makers Watson Pharmaceuticals (Corona, CA) and Par Pharmaceutical Companies (Woodcliff Lake, NJ) to delay generic competition to Solvay's branded testosterone-replacement drug "AndroGel," a prescription pharmaceutical with annual sales of more than $400 million, according to an FTC press release.

The US Pharmacopeial (USP) Convention has announced new standards for heparin and glycerin.

Brief pharmaceutical news items for February 2009.