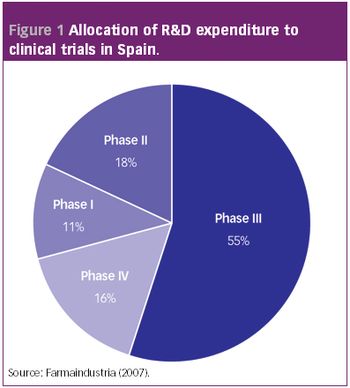
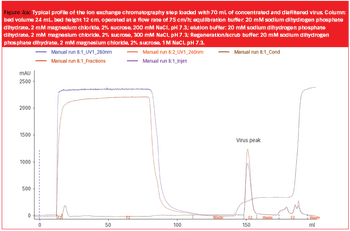
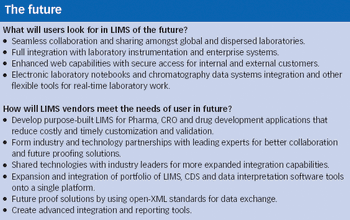
Pharmaceutical Technology Europe
How much do you know about parallel trade? Perhaps you may have heard someone mention these words and have then switched off. In a sense, it's hardly surprising given the fact that most media coverage centres on interpretation of complex legal cases. By the time you reach the end of these types of articles, you can't work out what the mentioned companies were arguing about in the first place and on which technical details the case was judged. Yet, time after time, a legal ruling on a parallel trade issue rockets to the front pages of the pharmaceutical press and even, occasionally, the mainstream media.