
An analysis of controlled correspondence between generic-drug manufacturers and FDA reveals patterns in questions about specific drug chemistry topics and the response to information provided in FDA guidance documents.
Thomas O’Connor is chemical engineer, science staff at the Office of Pharmaceutical Quality, Center for Drug Evaluation and Research, FDA.

An analysis of controlled correspondence between generic-drug manufacturers and FDA reveals patterns in questions about specific drug chemistry topics and the response to information provided in FDA guidance documents.
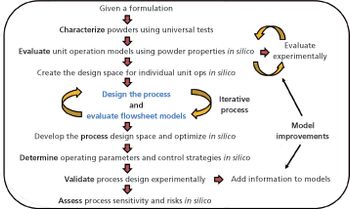
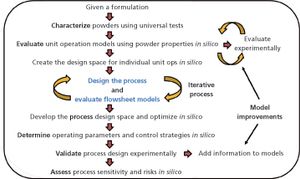
In-silico design facilitates process optimization and evaluation of process control strategies.
Published: April 2nd 2015 | Updated:

Published: September 1st 2015 | Updated: