
For the first time, cancer treatments accounted for 5% of overall drug spending in the first half of 2009, said Medco Health Solutions in a press release last week.

For the first time, cancer treatments accounted for 5% of overall drug spending in the first half of 2009, said Medco Health Solutions in a press release last week.

Formoterol presents formulators and manufacturers in the asthma and chronic obstructive pulmonary disease marketplace many challenges.

Teva Pharmaceutical Industries and Barr Pharmaceuticals (Montvale, NJ) signed a definitive agreement under which Teva will acquire Barr, the fourth-largest generic-drug company in the world. Teva will buy each share of Barr common stock for $39.90 in cash and 0.6272 Teva shares. Based on the closing price of Teva?s shares on July 16, 2008, the price per share of Barr common stock is $66.50, and the total value of the acquisition is $7.46 billion. Teva will assume Barr?s outstanding net debt of approximately $1.5 billion.

3M Drug Delivery Systems has successfully designed a proof of concept device using a solid microstructured transdermal system for the systemic delivery of high-potency pharmaceuticals. The technology was showcased at a poster session at the annual meeting of the Controlled Release Society held this week in New York City.

The US Food and Drug Administration advised patients, caregivers, and healthcare professionals to switch to hydrofluoroalkane-propelled albuterol inhalers now because chlorofluorocarbon-propelled inhalers will not be available in the US after Dec. 31, 2008.

What makes a drug ripe for respiratory delivery?

A microelectronic system based on radio-frequency (RF) cell ablation addresses limitations of other transdermal drug-delivery methods. This system expands the transdermal spectrum to include the delivery of water-soluble molecules, peptides, proteins, and other macromolecules.

For pediatric and geriatric patients, fast-dissolving drug-delivery systems provide an easier way to take medications and vitamins. Oral thin films have evolved to provide systemic delivery of active pharmaceutical ingredients for over-the-counter and soon, prescription drugs. The authors review the practical benefits of dissolvable films, their manufacture, and their market potential.

Scientists at the University of North Carolina at Chapel Hill have successfully tested a new inhaled tuberculosis (TB) vaccine.

Transdermal delivery takes up once-forbidden compounds, reviving markets and creating formulation opportunities.
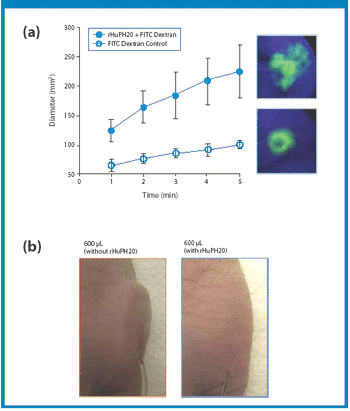
The preferred route of administration for an injected therapeutic agent is subcutaneous (SC), but SC injections are generally limited to no more than 1-2 mL in volume, representing a major challenge, especially for large protein biologics.

The book is written for drug-delivery scientists experienced in the dermatological or transdermal fields.
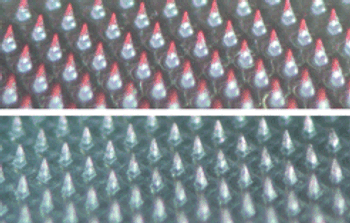
Following the sales of its global branded pharmaceutical business, 3M stays the course in drug delivery as its Drug Delivery Systems Division advances technology in inhalation and transdermal drug delivery.

Bringing Exubera to market requires extensive collaboration by Pfizer, Nektar Therapeutics, West Pharmaceutical Services Tech Group, and Bespak.

Bang & Olufsen Medicom (Copenhagen, Denmark, www.medicom.bang-olufsen.com), a drug-delivery device solutions provider, and Bespak (Milton Keynes, UK, www.bespak.com), a medical-devices and inhalation-valve technologies company, established an exclusive partnership to codevelop and comarket the ?Assist Actuated Inhaler,? a single-increment, dose-counting inhaler. The integrated device features an assisted firing mechanism for easier patient use, according to a company release.
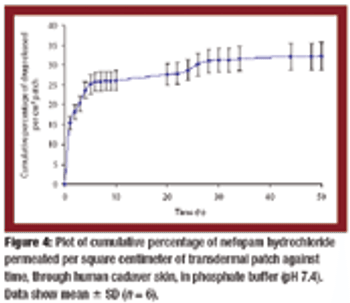
Transdermal matrix-type patches of Nefopam hydrochloride with a combination of pressure-sensitive adhesives were developed. The polymeric composition provided a controlled and sustained release of the drug from the patches and demonstrated favorable physicochemical characteristics.

BMS Receives FDA Approval for Transdermal Antidepressant


Transdermal drug technology specialists are meeting demands for methods that can painlessly deliver larger molecules in therapeutic quantities.

A new active dry powder inhaler has been developed using vibration frequencies to aerosolize powders.

New research has led to significant advances in formulations and inhalation systems for pulmonary delivery.

Transdermal drug delivery, a vital technology of increasing interest, offers such benefits as controlled delivery for as long as a week and improved patient convenience and compliance.

Recent advances in polymer science have resulted in a wide range of commercially available polymers for use in transdermal drug delivery systems.

A knowledgeable and experienced contract service provider can help in the development and success of an inhalation drug product.

Adhesives manufacturers can be involved in the development of transdermal drug delivery products by working with pharmaceutical companies on R&D, materials qualification, manufacturing, and other product development tasks.