
MHRA has been accepted as a full member of three international work-sharing partnerships aimed at making sure medicines and medical devices are regulated safely and efficiently across the world.

MHRA has been accepted as a full member of three international work-sharing partnerships aimed at making sure medicines and medical devices are regulated safely and efficiently across the world.

NICE has published its final draft guidance recommending the use of Vazkepa (icosapent ethyl) in adults to reduce heart attacks and strokes.

The draft ICH Q9(R1) document details the importance of quality risk management principles.

The new draft guidance describes a standards recognition program for regenerative medicine therapies.

In the report, the agency presents the activities it accomplished in 2021 to protect and promote public health.

The European Commission has granted conditional marketing authorization to the Janssen Pharmaceutical Companies of Johnson & Johnson for CARVYKTI (ciltacabtagene autoleucel), a cell therapy for treating multiple myeloma.

The agency’s Medicines Shortages Steering Group has adopted a list of critical COVID-19 vaccines and treatments so they may be monitored for potential shortages.

Roche has received approval from the European Commission for Lunsumio (mosunetuzumab), the first CD20xCD3 T-cell engaging bispecific antibody for treating follicular lymphoma.

FDA has approved GSK’s vaccine for the prevention of measles, mumps, and rubella in individuals 12 months of age and older.
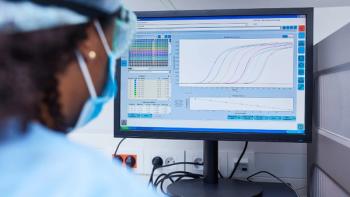
This article describes the in-vitro permeation test study data processing procedures and FDA statistical mathematics of evaluating a generic topical drug product, acyclovir cream, against its reference product.
When considering whether to outsource work to a CRO, there are a number of factors to assess, including the type of work that may be outsourced, regulatory considerations, and needs for the study.

EMA has been assigned a formal role in preparing for and managing future public health crises within the EU through the formalization of ad-hoc structures and processes that were employed during the COVID-19 pandemic.

FDA will use virtual site visits even after resuming active inspections.

Manufacturers are aligning with pharmacists and providers to blame high drug costs and limited patient access on pharmacy benefit managers.

FDA continues efforts to incentivize drug manufacturers to follow higher data integrity requirements.

FDA once again is taking steps to facilitate the import of less costly prescription drugs.

Dupixent (dupilumab), developed by Regeneron Pharmaceuticals in partnership with Sanofi, has received FDA approval for a new indication—treating eosinophilic esophagitis, a chronic inflammatory disease.

A new initiative aims to speed the approval of and access to new drugs for young patients around the world, while limiting the number of children needed for testing in clinical trials.

AstraZeneca's recombinant COVID-19 vaccine, originally invented by the University of Oxford, has been approved as a third dose booster vaccine in the EU.

EMA has recommended the marketing authorization of Xenpozyme (olipudase alfa) in the European Union.

PTC Therapeutics has received a positive opinion from EMA for Upstaza for the treatment of AADC deficiency.

The final guidance addresses safety aspects of container and carton labeling design.

The agency is asking drug manufacturers to ensure a strong supply chain by developing risk management plans.

FDA has approved Mounjaro (tirzepatide) injections as a treatment to improve blood sugar control in adults with type 2 diabetes.

Pfizer and BioNTech’s COVID-19 vaccine has been granted EUA for booster doses in children five through 11 years of age.