
For planned acquisitions or licensing, a careful analysis of CMCl factors is vital to ensure no problem areas are overlooked.

For planned acquisitions or licensing, a careful analysis of CMCl factors is vital to ensure no problem areas are overlooked.

Analysts should understand how a monograph, together with the associated general notices and general chapters, relate to their responsibilities under good manufacturing practices.

Ongoing and emerging cyber-risks to legacy operational technology systems in bio/pharmaceutical manufacturing must be addressed.

Building employee participation and forming good habits contribute to a company-wide quality culture that pays off.

Find links to pertinent regulatory and standard setting resources, guidance documents, and guidelines.

Oxular has been granted rare pediatric disease and orphan drug designations by the US FDA for its proprietary drug, OXU-003.

The UK's MHRA has joined the Australia-Canada-Singapore-Switzerland (ACSS) Consortium of regulators.

Bio/pharma industry leaders support FDA’s newly published EUA guidance for COVID-19 vaccine authorization.

The guidance provides recommendations for data and information needed to support an EUA for COVID-19 vaccines.

The agency sent an open letter to European Ombudsman Emily O’Reilly affirming the agency’s intention to apply the same standards to the evaluation of COVID-19 treatments as it does to other medicines.

The case for migrating from a paper-based quality management system to a digital platform is presented.

A comprehensive rewrite of Annex 1 has been proposed and aims to organize and structure requirements in 10 specific sections.
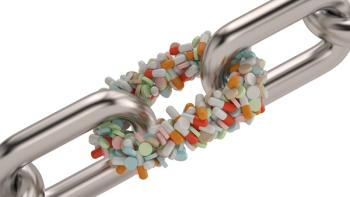
End-to-end traceability can provide more value than just securing drug product safety.

The agency published recommendations for the development of drugs and biologics for the adjuvant treatment of renal cell carcinoma.

Drug shortages and supply chain challenges bolster FDA efforts to promote modern manufacturing.

Regulatory bodies are adapting to new ways of working to cope with the impact of COVID-19.

The amount of detail included in SOPs may help a company stay compliant, says Susan J. Schniepp, Distinguished Fellow at Regulatory Compliance Associates, LLC.

The guidance discusses acceptable clinical endpoints for demonstrating effectiveness of drugs developed to treat opioid use disorder.

The European Medicines Agency has started a rolling review of AstraZeneca’s COVID-19 vaccine.

Symbiosis has successfully completed a scheduled inspection by MHRA.

The approval of Nucala (mepolizumab) for treating hypereosinophilic syndrome represents the first drug approved for this group of rare blood disorders in nearly 14 years.

With little more than a month to go until the national election, President Trump announced a revised initiative designed to reduce what consumers pay for prescription drugs, while also promising to protect coverage for pre-existing health conditions.

FDA and EMA have accepted the biologics license application and marketing authorization application, respectively, for bimekizumab.

A strategic serialization partnership has been announced by advanco and Syntegon, aimed at tackling the global issue of counterfeit drugs.

Telstar has reinforced its consultancy service to aid pharmaceutical companies in the compliance of the latest version of the EU GMP Annex 1 guidance.