
McNeil Consumer Healthcare is voluntarily recalling certain lots of over-the-counter products in the Americas, the United Arab Emirates, and Fiji.

McNeil Consumer Healthcare is voluntarily recalling certain lots of over-the-counter products in the Americas, the United Arab Emirates, and Fiji.

The United States Pharmacopeial Convention is recalling USP 33?NF 28.

The European Commission has asked certain pharmaceutical companies to submit copies of their patent-settlement agreements.

The US Food and Drug Administration released a warning to the public on Dec. 29, 2009 about criminals posing as FDA special agents and other law enforcement personnel as part of an international extortion scam.

Bioject Medical Technologies establishes alliance with MPI Research; Biogen Idec CEO to retire; And More.

Grace McNally, in the Office of Compliance at FDA's CDER, discusses the role of QbD and pharmaceutical quality systems as it relates to contract manufacturing.

Team leaders in FDA's Office of Generic Drugs provide an overview.

Plus, novel dosage forms and emerging trends.

Directors and staff miss the mark when it comes to following procedures.

This month sees Europe make its move towards electronic submissions harmonization, but is it ready?

The free movement of goods across Europe might be beneficial for some, but for pharmaceutical companies, the parallel trade of medicines is not welcomed, and is viewed as a system that can damage patient safety and innovation

BioNanomatrix (Philadelphia) names Edward Erickson president and CEO; and More.

Fraud and abuse in healthcare costs individual governments as much as $23 billion a year, according to estimates from the World Health Organization (WHO).

The European Public Health Alliance published its comments about the Falsified Medicines Directive currently under debate by the European Parliament and Council.

Do advanced therapies represent medical miracles or risky business? This is the question that is debated by an Informa analyst in an Executive Briefing for Scrip.

The US Pharmacopeial Convention posted a proposed revised standard for what should and should not appear on the ferrules and cap overseals of medication vials.

Pharmaceutical Technology talks to FDA's Justina A. Molzon about current discussions with international regulators, and how, after 20 years of harmonization activities and the common technical document, ICH has benefited regulatory authorities.
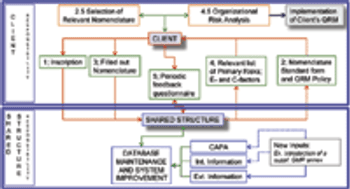
Representatives to the PIC/S provide an example of methodology for implementing ICH Q9.

Dumbed-down presentations and poor speaker selections are destroying a valuable industry tool.

With so many healthcare and pharmacy websites, consumers could use the agency's nod of support.

The authors discuss a study that demonstrates the use of polyethylene oxide mixtures to investigate the effect of polymer viscosity on formulation robustness.

Blame it on cafeteria gossip, outdated procedures, and major miscommunication.

Generic-drug manufacturers look to expand into biologics and complex dosage forms. This article contains bonus online-exclusive material.
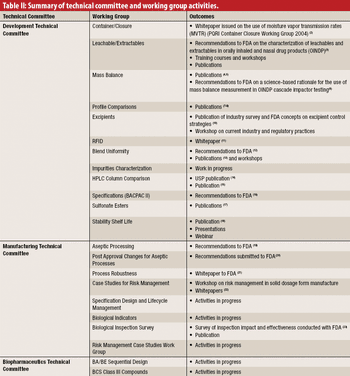
The authors describe the development and organization of the Product Quality Research Institute and highlight some of the important projects conducted by its Working Groups.

The quality assurance environment is forcing pharmaceutical companies to face new challenges. In light of this, the authors conducted a study of professionals from some of the world's top pharmaceutical companies to identify key QA concerns.