
EMA has recommended approval of the biosimilar for the same indications as Roche’s Herceptin (trastuzumab).

EMA has recommended approval of the biosimilar for the same indications as Roche’s Herceptin (trastuzumab).

Telstar reports that its Boreas, a new 86° ultra-low-temperature freezer, increases average performance by 20%.

The agency’s Committee for Medicinal Products for Human Use recommended six drugs for approval at its March 2018 meeting.

The draft guidance addresses the agency’s policy on evaluating bulk drug substances in drug compounding.

The agency’s Clinical Data Summary Pilot program will post redacted Clinical Study Reports in order to help stakeholders understand why FDA approved a new drug application.

The agency sent a warning letter to Malladi Drugs & Pharmaceuticals Limited after an inspection found CGMP violations that included the presence of vermin.

The agency published two new guidance documents detailing postmarketing safety reporting requirements for combination products.

The European Medicines Agency has published a new tool that gives a transparent overview of the agency’s relocation to Amsterdam.

Sartorius Stedim Biotech has launched a new automated parallel bioreactor system for perfusion culture.

The agency announced proposed research studies on how healthcare providers and patients understand drug promotional materials.

The delivery device and drug form should be considered when choosing a test method for identifying and measuring particulates.

A previously published article presented difficulties with the revised European guidelines on sterile manufacturing. The authors included a brief summary of the comments developed on the draft document. This article expands upon that summary, outlines the authors' rationale, and highlights the most difficult aspects of the revision draft.

Revisions to chapters on glass containers and elastomeric closures were canceled following review of comments.

The European Medicines Agency has called for the immediate suspension and recall of AbbVie and Biogen's multiple sclerosis drug Zinbryta (daclizumab beta).

The FDA commissioner outlined the agency's initiatives to reward innovation and biosimilars development.

With minimal damage to its manufacturing plant in Humacao, Puerto Rico, Colorcon has been able to restore full operations.

The agency has approved a new HIV treatment for patients with "limited treatment options".

This article presents considerations for preventing hazards associated with dust in pharmaceutical production, particularly as relates to the United Kingdom's Control of Substances Hazardous to Health (COSHH) Regulations.
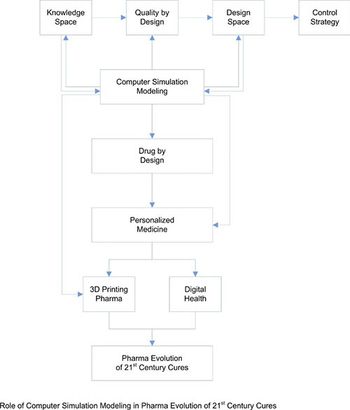
Twenty-first century drug development challenges sponsors and contract partners to collaborate more closely, using tools such as model-based simulation.

The company is recalling Methylprednisolone Sodium Succinate for Injection, USP, 40mg, 125mg, and 1g manufactured by Gland Pharma Ltd and distributed by Sagent due to out-of-specification impurity results.

The company is recalling three lots of Hydromorphone HCl Injection USP CII 10 mg/mL, 1 mL in 2 mL Single Dose Vials because of possibly empty or cracked vials.

The upcoming serialization deadline and the United Kingdom's departure from the European Union could result in supply bottlenecks.

This article describes a new, combined, quantitative method for assessing excipient risks that has been developed by the authors as one possible risk evaluation method.

Manufacturers face demands for timely information on clinical studies, product recalls, and approvals.

In-house experts can help select the right systems and suppliers, making validation and compliance easy, says Siegfried Schmitt, principal consultant at PAREXEL.