
When assessing the competencies of an outsourcing service provider, sponsor companies must pay a great deal of attention to the cGMP compliance level of the provider.

When assessing the competencies of an outsourcing service provider, sponsor companies must pay a great deal of attention to the cGMP compliance level of the provider.

Week of Aug. 23, 2010: Company and People Notes: Quark and Novartis Form Agreement; Hospira CEO Retires; And More.

US Department of Health and Human Services (HHS) Secretary Kathleen Sebelius released last week an examination of the federal government's system to produce medical countermeasures, or medications, vaccines, equipment, and supplies needed for a health emergency.

Regulatory Roundup: PDUFA Fees Published, And More.

GlaxoSmithKline Consumer Healthcare, Cephalon UK Ltd, and ProStraken Group plc have been accused of breaching the Association of the British Pharmaceutical Industry's (ABPI) Code of Practice.

The US Food and Drug Administration and European Medicines Agency (EMA) are looking for drug manufacturing companies to participate in their joint good manufacturing practice (GMP) inspection pilot program.

Commissioner Hamburg Says China is Improving Export Regulation

Changing or upgrading cleanroom gloves requires time and due consideration. A good decision could improve employee satisfaction and product yields, but a bad decision could necessitate millions of dollars worth of rework, recalls, and rejects if the gloves don?t perform as expected. Personnel should consider various criteria to choose the best glove for their cleanroom.

Novartis has been told off by the FDA in a letter about a social media Facebook widgit that appears on the company?s Tasigna website.

In a draft guidance published this week, the US Food and Drug Administration recommended that makers of transdermal and transmucosal drug-delivery systems use "an appropriate scientific approach" during product design, development, and manufacturing to minimize the amount of residual drug substance present at the end of the products' labeled use periods.

FDA Approves Flu Vaccines; Caraco Names COO; And More.

Legislative efforts to modernize provisions in the Toxic Substances Control Act of 1976 (TSCA), which involve chemical safety and reporting requirements is drawing criticism from the chemical industry.

The Senate Appropriations Committee approved a measure last week that would restrict the use of certain patent settlements by innovator-drug companies under which innovator-drug companies pay generic-drug companies to delay the entry of a generic product in so-called "pay-for-delay" cases.

Industry's focus on cost cutting has led to a dangerous gap in training and knowledge.

The authors discuss the statistical tools used in experimental planning and stategy and how to evaluate the resulting design space and its graphical representation.

Cases of overlooking proper packaging, reconstitution, directions, and dissolution.

FDA chemistry reviewers in the Office of Generic Drugs provide an overview of common deficiences cited throughout the Chemistry, Manufacturing, and Controls section of ANDAs.

Manufacturers and regulators on both sides of the ocean move to ensure the safety of heparin and other globally distributed drug products.

Industry can meet its responsibility to society by considering innovative pricing and partnerships.

Three therapies under review could help Americans attain a healthy weight.
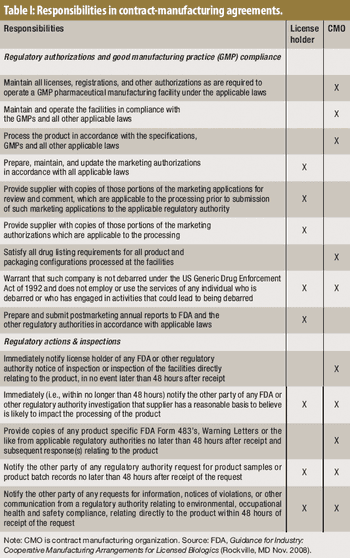
Before embarking on an outsourcing relationship, it's important to be aware of FDA's expectations.

The different pathways to regulatory approval of a biosimilar vary worldwide, ranging from no pathways at all in some developing countries, to the complex and precise mechanism that exists in Europe.

A new guideline for conducting bioequivalence studies was adopted by the CPMP in January and it becomes fully effective as of August 2010.

We speak to The European generic Medicines Association about the environment for biosimilars.
