
To ensure the accuracy of scientific testing, protect the subjects to avoid data contamination.

To ensure the accuracy of scientific testing, protect the subjects to avoid data contamination.

White House and Congress likely to struggle over funding for bio/pharmaceutical regulation.

Can postapproval FDA filings immunize pharma companies from patent lawsuits?
A change in terminology could emphasize patient protection.

Domestic companies are changing their business models in response to recent drug price cuts.

EMA addresses drug supply shortage.

Overt and covert packaging technologies have evolved to authenticate drugs and fight counterfeits.

FDA announced the availability of the guidance “Q11 Development and Manufacture of Drug Substances†in the Nov. 20, 2011 edition of the Federal Register.

USP announces new drug naming policy and FDA releases two guidance documents.

FDA provides information on its Enhanced Communication Team created in response to PDUFA V.

An operation spanning 16 African countries and conducted by the World Customs Organization (WCO) in partnership with the Institute of Research against Counterfeit Medicines (IRACM) led to the seizure of more than 82 million doses of counterfeit medicines.

FDA pushes back goals due to Hurricane Sandy and EMA announces changes to variation regulations.

USP revises labeling requirements for Heparin.

At the inaugural joint FDA/ISPE conference on CGMP earlier this year, CDER Dirctor Janet Woodcock delivered a strong message to the pharmaceutical industry: the efforts to adopt QbD principles must be a top priority.

Manufacturing and in-depth characterization provide basis for demonstrating product equivalence.

Even when all is well at the facility, one must expect the worst while braving the elements.
The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on biosimilar monoclonal antibodies (mAbs).

The government of Turkey is drawing up a program in coordination with the pharmaceutical industry to create ways to make the country a regional production center for pharmaceuticals serving Europe, Central Asia, and the Middle East.

The US Pharmacopeia's revised General Chapters on elemental impurity limits and testing procedures are set to take effect in December 2012.

The Pharmaceutical Inspection Co-operation Scheme (PIC/S) has finalized a risk-based inspection planning tool for inspectorates to use in applying science- and risk-based principles to planning GMP inspections.
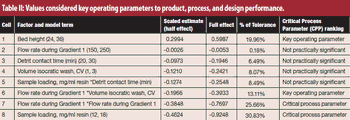
Critical process parameters (CPPs) and their associated process controls are crucial to drug development and process validation and to the evaluation of every manufacturing unit operation.
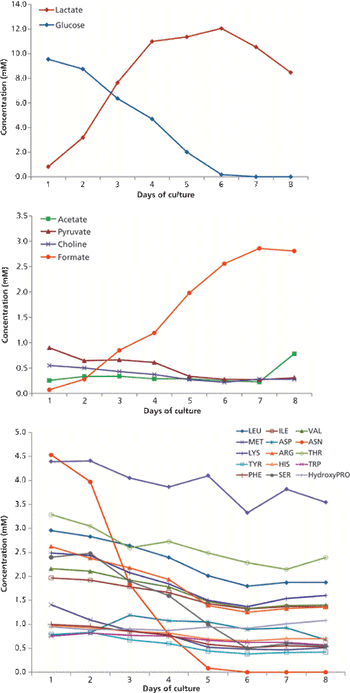
The author discusses current expectations in bioprocessing and lays a framework for using NMR to enhance a QbD approach.

New England Compounding Center gets 483 after linked fungal meningitis outbreak.

FDA announces Coalition for Accelerating Standards and Therapies and Commissioner Hamburg comments on meningitis outbreak.

EMA releases guideline on medicinal products for the treatment of schizophrenia.