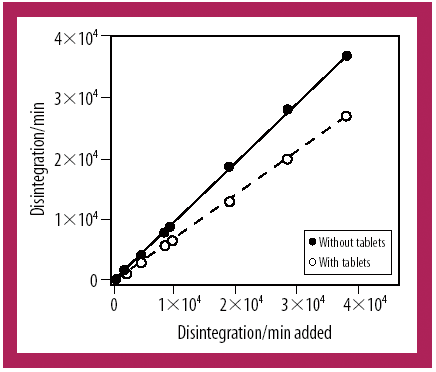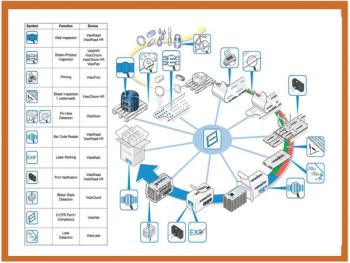
Adding Braille to pharmaceutical packaging should be less of a challenge with the use of Esko-Graphics' Scope solution. EC Directive 2004/27/CE requires Braille labelling and information to be provided with pharmaceutical products for human use, and companies are scrambling to implement this by 31 October.