
Company and People Notes: Cambridge Major Labs acquires ChemShop; Helix BioPharma restructures senior positions, more.

Company and People Notes: Cambridge Major Labs acquires ChemShop; Helix BioPharma restructures senior positions, more.

Company and People Notes: BASF raises prices on excipients; Verus Pharmaceuticals appoints president and CEO; more.

Pfizer's Lipitor patent revoked in Germany, promotions at Charles River Labs, more.

Wyeth Consumer Healthcare launched a voluntary recall and replacement program for US retail outlets that sell several ?Robitussin? and ?Children?s Dimetapp Cold and Chest Congestion? products.
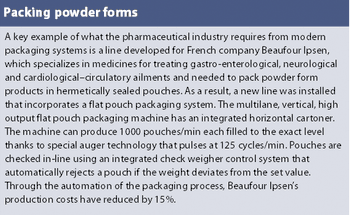
Pharmaceutical manufacturers are under increasing pressure to shorten time-to-market, produce treatments with unpredictable product lifetimes, provide greater flexibility and, at the same time, comply with ever more stringent quality, validation, stability and traceability constraints. While this is encouraging for the contract manufacturing sector, it creates the need for even greater manufacturing flexibility.

Company Notes: Gibraltar expands facilities; Allozyne appoints new president and CEO, more.

Company and People Notes: ImClone, BMS, and Merck form agreement; CRI Worldwide names new CEO, more.

Company and People Notes: Orexo acquires Biolipox, Avalon cofounder resigns, more...

Company and People Notes: Novartis and MIT to study continuous processing, GSK appoints Andrew Witty as CEO, more.

Company and People Notes: Thermo Fisher Scientific buys Priority Solutions International, Wyeth elects new president and CEO, more.

When it comes to designing and sourcing sustainable packaging, there are no simple answers. Sustainable packaging depends on a complex interaction of environmental, social, and economic considerations, which are influenced by geography and other factors such as renewability, compostability, biodegradability, weight, and performance.

Nonpharmaceutical manufacturers find ways to make environmentally sound packaging.
Security, the environment, ageing populations, the bio-boom and cost control are just a few of the drivers that will influence pharmaceutical packaging for the remainder of this decade.

Company and People Notes: Evotec and Renovis enter agreement, Amgen to lay off 675 workers, more.

Company and People Notes: Catalent expands Bolton, UK, warehouse; Biotica appoints Edward E. Hodgkin as CEO and director; more.

Company and People Notes: Baxter and Halozyme Expand Relationship, Crucell Names COO, More.

Going digital can produce high-quality, full-color labels at potentially lower cost.

Makers of temperature-sensitive products constantly seek to ensure proper conditions during shipping and storage.

The company begins production at a new $100-million manufacturing facility for prefilled injection systems, plans further investment in packaging facilities, and targets both early-phase development and commercial manufacture.

Drug packaging performs functions such as ensuring patient well-being, providing information, preventing tampering, blocking counterfeiting, and improving compliance. Since 1977, packaging innovations have occurred in four major categories. The author provides an overview of major packaging improvements that have emerged in the past 30 years.

Drug companies have come to realize that spending heavily on creating new blockbuster drugs is risky and less cost-effective ...

Exhibitors' products prevent counterfeiting, provide child resistance, protect product quality, and improve packaging-line efficiency.

Many radio frequency identification projects are moving beyond the pilot stage, supported by new hardware and software tools.

Security, the environment, aging populations, the bio-boom, and cost control are just a few of the drivers that will influence pharmaceutical packaging for the remainder of this decade.

A security technology, previously exclusively reserved for use by government organizations has become commercially available with major pharma manufacturers eager to adopt the device.