
Also, PDL BioPharma will no longer pursue sale of the company, executives resign from Topigen Pharmaceuticals, more...

Also, PDL BioPharma will no longer pursue sale of the company, executives resign from Topigen Pharmaceuticals, more...

Also, Millipore plans to open Singapore facility, Michael J. Simms joins Alexza Pharmaceuticals, more...

New packaging options monitor and protect temperature-sensitive products.

Show blasts off this month in Philadelphia with more suppliers, new trends, and real-world solutions.

Brief pharmaceutical news items for March 2008.
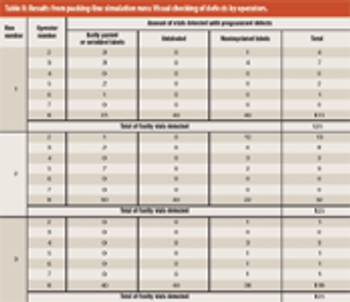
This article focuses on upgrading and improving a packing process to comply with current good manufacturing practices. The authors sought to maintain proper quality assurance for finished products.

Twenty years ago it was commonplace for pills, tablets and capsules to come in small, plastic or even glass bottles. Syrups were a much more common galenic solution than today, and individual dosages of injectables were only offered in glass vials and ampoules.

Also, Genmab will acquire PDL Pharma's Minnesota manufacturing facility, DSM Pharmaceuticals appointed Hans Engels president and business unit director, more...

Also, Biocon will acquire 70% of AxiCorp, ARIAD Pharmaceuticals promoted Richard W. Pascoe to the new position of COO, more...

Chesapeake to relocate and expand, AVI BioPharma appoints CEO, More...

Amira and GSK Form Agreement, Xenome Appoints Ian Nisbet CEO, More...

PharmTech's polls feature user feedback on issues facing the pharmaceutical industry.

A news roundup for February 2008.

Can an overload of patent applications lead to the US' demise as a scientific leader?

Jacketed hose provides cleanability; Stopper prevents product waste; Accessories control temperature precisely

Members of Congress have strongly urged the US Food and Drug Administration to reconsider its proposed rule to amend regulations permitting companies to promptly update their drug and device labels with new safety information.

Codexis Opens Budapest Lab, Allos Names Bruce K. Bennett VP of Manufacturing, More...

Actavis Acquires Site From Pfizer, Cardiokine Names President and CEO, More...

Novo Nordisk Drops Inhaled Insulin Product, Neurocrine Appoints CEO and President, More...

Crucell and Sanofi Pasteur Sign Agreement, WuXi Pharma Names COO, More...

Merck Sells Facility to Cherokee Pharmaceuticals, Inflazyme Announces Senior Management Resignations, More...

Brief pharmaceutical news items for January 2008.

Editors' Picks of Pharmaceutical Science & Technology Innovations

Millipore and Novozymes Form Alliance, Applied Biosystems Names President and CEO, More

Company and People Notes: West to reduce workforce, Eli Lilly's CEO and chairman to retire, more...