
Virtual pilot programs that simulate scenarios can help the pharmaceutical industry address core issues in the implementation of serialization systems that comply with the US Drug Supply Chain Security Act.

Virtual pilot programs that simulate scenarios can help the pharmaceutical industry address core issues in the implementation of serialization systems that comply with the US Drug Supply Chain Security Act.

Citing failure to meet child-resistant closure requirement, Merck has advised that all bottles should be examined for cracks and that affected bottles should be kept out of children’s reach.

Ger Standhardt, manager of knowledge development & projects at NVC, shares insights on the role of pharmaceutical packaging and accessible design in patient adherence.

WuXi PharmaTech and TruTag Technologies successfully applied and detected TruTag's edible microtags on solid-dosage drug products and found no effect on dissolution.

Wourld Courier's Climate Optimisation Research & Engineering (CORE) Lab near Cologne, Germany, will enhance package evaluation and validation.

Choosing the correct shipping solutions, including packaging, transportation mode, and monitoring, helps mitigate the risks inherent in global logistics.

Create an efficient global labeling strategy that is compliant with both electronic and paper-based package-insert requirements.

CSafe and AES will support controlled-temperature shipping through the Basel, Switzerland airport from their new service center.

Checkweighers, metal detectors, x-ray inspectors, leak detectors, headspace analyzers, and optical inspection systems for packaging were demonstrated at INTERPHEX 2015.

Sepha's VisionScan Max can leak-test full production batches of blister packs.

Electronic pharmaceutical tablet counters meet demands for accuracy, flexibility, speed, compact size, easy cleanability, and quick changeover.

PTI Inspection System’s VeriPac 310 leak detection and package integrity testing system reduces waste and provides the user with a clear evaluation of package integrity.

The agency gives a limited reprieve to dispensers but requires other trading partners to provide product tracing information.

Pharmacy associates say more education regarding the transactional requirements of DSCSA is needed.

The agency clarifies its requirements for allowable excess volume and labeled vial fill size in injectables and biologics.

Although full traceability is not required by law in the US until 2023, companies could benefit from implementing it now.
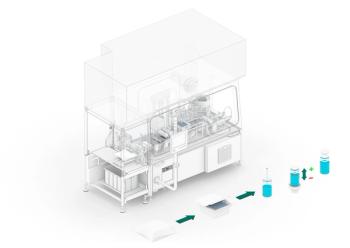
Groninger has developed FlexPro 50, an isolator system for aseptic processing of nested syringes, cartridges, and vials. FlexPro 50 is capable of achieving outputs of up to 5000 containers per hour.

The increasing number of biopharmaceuticals in the development pipeline has created the need for packaging technologies with enhanced safety features, especially for sensitive biologic drugs.

Pioneering system provides induction sealing integrity analysis for 100% of bottles without packaging line slowdown.

Schubert-Pharma is featuring the world's first prototype of a packaging machine without an electrical cabinet.

Domino’s G-Series thermal ink jet, D-Series laser coders, and V-series thermal transfer printers have been developed to help manufacturers comply with new global serialization and traceability legislation.

Herma’s 132M HC labelling machine is suitable for applications that require high-performance wrap-around labelling.

Technologies for serialization and aseptic packaging are being displayed at the show.

Machine manufacturer IMA is showcasing a number of technological advances for the processing and packaging of pharmaceutical products at ACHEMA 2015.

The Hapa 862, from Hapa AG, is a modular, CMYK/spot-color inkjet printing system for the in-line packaging printing of foils and labels.