
Despite challenges, contract manufacturers in Europe are enjoying considerable success.

Despite challenges, contract manufacturers in Europe are enjoying considerable success.

Pharma companies could benefit from the lessons learned in this fall's financial crisis.

Pharmaceutical Technology will feature video coverage of AAPS this month.

More than 235 biotechnology companies call New Jersey home. They range from early start-ups with just a few employees to fully functioning companies with research, marketing and sales offices.

Although California remains the country's biotech leader, Michigan anticipates that the wind is shifting direction, albeit slowly

Richard Kilner, Managing Director of the Commonwealth of Pennsylvania European Investment Office, is incredibly enthusiastic about both his State and the potential it offers investors: "We are the number one State in pharmaceuticals (by number of establishments, employment and GDP output) and rapidly closing in on Massachusetts, the number two in biotech."

Together, Europe and the US account for more than 70% of the global pharmaceutical market, and the growth of these markets is heavily dependent on distribution systems.

Access to the capital markets of the US has always been a key attraction for Europe's biotech businesses.

California's forward-thinking reputation, well-funded research universities and world leadership in the potentially life-saving field of stem cell research and green energy provide a progressive and positive business environment for biotech companies.

Speaking to Pharmaceutical Technology Europe, Georgia Bio's Director of Innovation and Technology, Carol Henderson, outlined the unique assets that make Georgia an attractive location for international bioscience companies.

According to Deputy Director, Alexander Bothmann, of Enterprise Florida Inc., in Germany, the 'Sunshine State' is: "Committed to building a world-class biotechnology sector by investing in research facilities, fostering the growth of local biotech companies and welcoming progressive newcomers, such as the Scripps Research Institute, the Burnham Institute for Medical Research and Torrey Pines Institute for Molecular Studies."

Also, Maxygen looks to costs, jobs; Receptor BioLogix appoints Dale R. Pfost CEO; more...

Also, MedImmune opens Cambridge, UK, facility and makes reverse engineering pact with Omninvest; BD Medicine appoints Carol Adiletto VP of clinical and regulatory affairs; more...

Also, Millipore opens new membrane-casting manufacturing facility in Ireland; Surface Logix appoints Keith Dionne president, CEO, and a member of the board; more...

Rep. John D. Dingell (D-MI), chairman of the US House of Representatives Committee on Energy and Commerce, and Rep. Bart Stupak (D-MI), the chairman of the Oversight and Investigations Subcommittee, sent a letter to the US Department of Health and Human Services (HHS), questioning the US Food and Drug Administration?s use of agency resources to hire an outside public-relations firm to create a positive public image of the agency.

Also, Merck & Co. discontinues development of its obesity drug taranabant; Synthetech names Frederic Farkas director of manufacturing; more...

Buoyed by increased demand for pharmaceutical outsourcing services, Ricerca Biosciences proceeds with an expansion plan.

Summer deals struck by Novartis and Lilly point to an evolution in contract services.

Advancements add yet another challenge for industry's already overextended regulatory body.

Emerging pharmaceutical companies represent an important client base for CROs and CMOs. Lessons learned for successful customer–supplier relations.
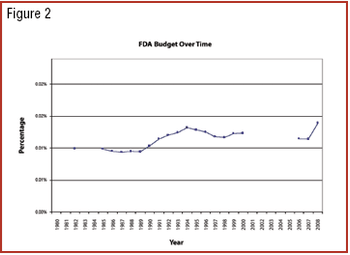
Can previous trends of Democratic and Republican administrations predict industry's future?

As offshore savings decline, pharmaceutical companies still have a lot of work to do to reduce costs.

Also, Alpharma advises shareholders to reject King's offer; ImClone rejects raised BMS offer; Immunogen appoints Daniel M. Junius, more...
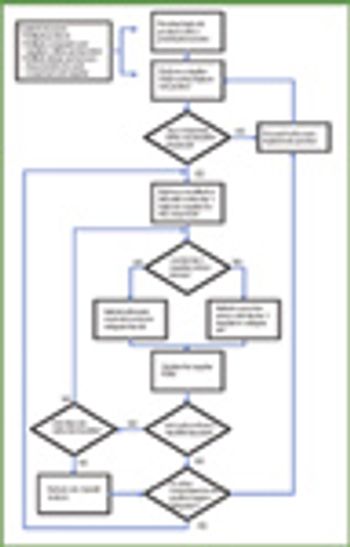
The growth and globalization of the pharmaceutical supply chain make risk assessment more important than ever for pharmaceutical manufacturers. The authors describe a program to identify, prioritize, mitigate, and communicate risks in manufacturer–supplier relationships.

The authors provide detailed lists of important checkpoints to consider when selecting an outsourcing provider.