
Big Pharma's efforts to reduce working capital will hurt CMOs for years to come.

Big Pharma's efforts to reduce working capital will hurt CMOs for years to come.

The end of 2009 is creeping closer and analysts are already looking towards 2010 and wondering what new challenges are in store for the pharma industry.

Company and People Notes: Merck KGaA to build R&D hub in Beijing; Depomed appoints VP of operations; more...
View Jim Miller's presentation from the AAPS 2009 Meeting and Exhibition.

Company and People Notes: Genzyme provides updates on enzyme replacement products and FDA complete response letter; Watson appoints VP of global operations; more...

Company and People Notes: 3M forms agreement with VaxInnate; TRIN Pharma appoints CEO; More...

Lonza (Basel, Switzerland), the large contract manufacturer of small-molecule and biologic-based active pharmaceutical ingredients (APIs), announced last week a series of cost-cutting measures for the next 12 to 18 months.

Company and People Notes: sanofi aventis forms pact with Micromet; Laureate Pharma adds members to its business team.

A panel of industry and regulatory experts, including FDA, discuss efforts to secure the global pharmaceutical supply chain. This article contains bonus online-exclusive material.

Contract organizations waiting for the pipeline to come roaring back are kidding themselves.

While Congress debates hundreds of healthcare plan proposals, perhaps we, the public, can get in the game too.

Engels discusses the latest industry developments and trends.

FDA's Edwin Rivera-Martinez on dodging supply-chain challenges.

New pricing controls and healthcare reforms may be pushing the pharmaceutical market out of this southeast Asian country. This article contains bonus online-exclusive material.

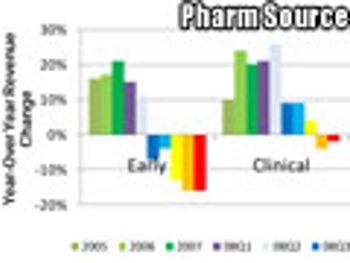
Apopular definition of insanity is repeating the same task over and over again while expecting a different result. It would appear, therefore, that many people in the contract services industry think the major global bio/pharma companies are insane.

The House Energy and Commerce Committee approved H.R. 2868, the "Chemical Facility Anti-Terrorism Act of 2009," a measure to modify and codify existing requirements of the Chemical Facility Anti-Terrorism Standards (CFATS) program.

Also, Astellas and Medivation sign development pact; WuXi PharmaTech appoints VP of business development, more...

US-based CRO, Health Decisions, hopes to raise the efficacy standards for complex studies and help companies get products to market faster by establishing an international network of CROs.

Company and People Notes: SurModics forms agreement with Roche and Genentech; Hospira names Daphne Jones senior VP and chief information officer; more...

Company and People Notes: Bayer Schering Pharma forms collaboration with Compugen; Takeda San Francisco appoints VP of process science; more...

Company and People Notes: GSK forms joint venture with China-based Jiangsu Walvax Biotech; Sigma-Aldrich appoints VP and board member; more...

AAPS President offers hope and solutions for the industry's challenging future.

Pharmaceutical companies in India have had a hold on the biotechnology sector for many years, and they're not about to let the follow-on biologics market pass them by.

The countries of Central and Eastern Europe and the Commonwealth of Independent States are closing in on global pharmaceutical competition.
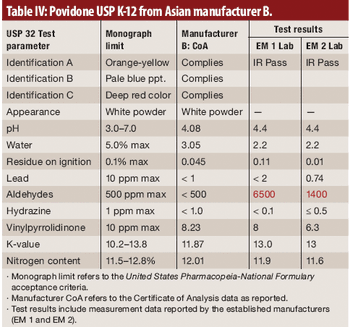
This article demonstrates that test results support the position of FDA on the importance of an appropriate supplier-qualification program.