
Pressures to accelerate current and next-gen therapies are challenging traditional microbiological testing models.

Pressures to accelerate current and next-gen therapies are challenging traditional microbiological testing models.

Dedicated facility will address enhanced regulation of metals and impurities in pharmaceuticals.

Idifarma has announced a growth of more than 50% over the past two years. Revenue is expected to hit approximately EUR5.3 million in 2016, growing from EUR3.5 million in 2014.

The new facility will focus on formulation development, drug product analytical development, and quality control.

CMO executives are focusing on M&A activity, new business models, and fundraising limits.

Recipharm is investing more than EUR1.2 million to enhance its small-scale GMP API development and manufacturing capabilities in Paderno Dugnano, Italy.

Catalent invests $34 million to add a 2 x 2000-L single-use bioreactor system and laboratory space in Madison, WI.
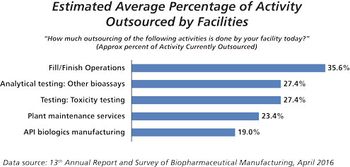
This key bioprocessing segment is expecting continued growth

The company announced that an August 2016 FDA inspection of the company’s facility resulted in no form 483s.

Saneca Pharma is making significant investment in its API capabilities to support client demand for smaller batch sizes and streamlined scale-up.

The strategies of a innovation-driven CMO may be different than a capacity-driven CMO.

Innovative technologies and services meet needs for existing and emerging biologic-based therapies.

The new facility is solely dedicated to offer extractables and leachables (E&L) testing services to the pharmaceutical and related industries.

The CordenPharma Chenôve, France manufacturing facility completed an FDA Inspection with no 483s reported.

API and drug product manufacturer changes name to align with parent company.

FDA found no observations during recent inspection of Regis Technologies manufacturing site.

Evonik’s parenteral drug delivery and commercial drug product manufacturing in Alabama passes EU GMP inspection.

The agreement comprises technology transfer and commercial manufacturing of Tillotts’ gastroenterology products.

Recipharm is investing EUR3.7 million (approximately $4.18 million) to increase lyophilization capacity at its Masate facility in Italy.

AMRI entered into a non-exclusive license agreement with the Broad Institute for the use of CRISPR-Cas9 gene-editing technology.

Fresenius Kabi will add to its generic, sterile injectable manufacturing at its Melrose Park, Illinois site.

Piramal Enterprises has entered into an agreement to acquire 100% stake in Ash Stevens all by cash for a consideration of $42.95 million plus an earn-out consideration capped at $10 million.

Agilent Technologies announces plans to build a new oligo manufacturing facility in Colorado that will double current capacity.

Quebec, Canada-based contract development and manufacturing organization has joined the Pharma & Biopharma Outsourcing Association.
The Claristep filtration system from Sartorius improves preparation of samples for analytical testing.