
Process optimization improves process mass intensity, reduces environmental impact, and improves cycle time for bio/pharmaceutical processes.

Process optimization improves process mass intensity, reduces environmental impact, and improves cycle time for bio/pharmaceutical processes.

The new facility, located at the company’s headquarters in Pittsburgh, PA, is expected to meet all clinical and commercial development needs of the company’s lead gene therapy program.

Iksuda Therapeutics and Femtogenix, have signed a license agreement aimed at progressing Iksuda’s lead ADC to clinic, with the aim of targeting difficult-to-treat solid tumors.

The companies will use GeoVax’s vaccine technology to develop malaria vaccine candidates.

Sartorius Stedim Biotech (SSB) and Novasep will partner to develop systems for membrane chromatography using Novasep’s BioSC platform and SSB’s single-use technology.

FDA guidance on quality considerations for continuous manufacturing should help advance implementation by providing clarity and giving companies more confidence to make investments in the new technology.

The Tandem 4.0 augmented reality-enhanced collaboration tool from Apprentice.io has a new user experience and user interface design.

AST will unveil its multi-format robotic filling system, the GENiSYS R, at INTERPHEX.

The Marchesini Group will introduce the CMP pharma inspection system to the US market at INTERPHEX 2019.

The company is voluntarily recalling four lots of Drospirenone and Ethinyl Estradiol Tablets, USP because of the possibility they may contain defective blister packs and incorrect tablet arrangements.

Identify the warning signs and follow best practices for refurbishment to improve tablet press yields.

New technologies in the digital factory enhance quality, efficiency, and flexibility for bio/pharmaceutical manufacturing.

HPLC coupled with charged aerosol detection is a suitable analytical technique to quantitate and characterize polysorbate-80 in therapeutic products; allowing quality assessment of raw material, content confirmation during manufacturing, and monitoring product stability.

A monoplant may offer greater supply security and flexibility for specialist medicines.

Containment valves and smart monitoring can keep employees safe and improve manufacturing efficiency when handling potent APIs, intermediates, and solid-dosage drugs.
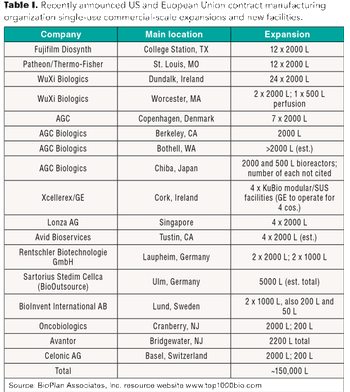
Single-use systems can be a cost savings for CMOs, and these savings can be passed on to clients and, ultimately, to patients.

While allogeneic therapies can use existing regulatory and quality frameworks, autologous treatments will require pharma’s adoption of true just-in-time and right-first-time concepts, says consultant James Blackwell.

Optical coherence tomography can improve quality control and development of coated dosage forms by allowing film thickness to be measured in real time.

The Pulse-Flow PTA dense phase pneumatic conveying system from Gericke USA directs air or nitrogen into the pressure vessel and pipeline in timed, regular pulses that form the dry material into separate, wavelike, pulsed slugs.

The VMC VersaMix by Charles Ross & Son Company is suited for temperature-critical formulations of medium to high viscosity under stringent vacuum/pressure constraints.

Eli Lilly and Company experts share the vision and the value of the digital plant for pharmaceutical manufacturing.

System connectivity and data analysis drive increased productivity in pharma manufacturing.

GHO Capital, a European specialist investor in healthcare, announced its acquisition of Sterling Pharma Solutions, which specializes in complex and difficult-to-manufacture APIs.

The Coalition for Epidemic Preparedness Innovations (CEPI) and CureVac partner to develop a transportable mRNA vaccine manufacturing platform.

The $21.4-billion acquisition will create stand-alone business within Danaher’s life-sciences portfolio.