
Equipment and systems for aseptic transfer and manufacture meet cGMP and Annex 1 requirements for pharmaceutical fill and finish.

Equipment and systems for aseptic transfer and manufacture meet cGMP and Annex 1 requirements for pharmaceutical fill and finish.

Digital tools, such as building information modeling (BIM) software, improves efficiency for optimizing pharmaceutical facility design.

At-line NIR measurements can replace a laboratory HPLC measurement for API content in oral solid-dosage drug manufacturing.

In this interview, the main uses and benefits of NIR spectroscopy in tableting are discussed.

Early adopters of continuous manufacturing approaches shared their plans and some of their experiences at the 11th annual Charles Jarowski Symposium in Industrial Pharmacy.

The editors welcome technical article contributions from the bio/pharma industry.

Front-end focus and new approaches are speeding scale-up and reducing costs, while scale-down and scale-out become increasingly important.

The evolution of cell-culture technology is driving the need for improvements in modeling solutions.

Oval Medical Technologies (Oval) has revealed that it is relocating to a specifically designed facility in Cambridge Research Park in the United Kingdom as a result of growth.

Alvotech and Prestige Biopharma, have announced the formation of a new contract manufacturing partnership for the commercial production of a biosimilar.

A collaboration between Insilico Biotechnology and IFAT aims to develop a manufacturing, planning, and control system for the production of monoclonal antibodies.

A new defined coated cell cultureware by denovoMATRIX allow for the serum-free expansion of mesenchymal stem cells.

The partners will collaborate to identify the bioactive components in hydrolysates, which have long been used as additives in cell culture media and feed formulation in the biopharma industry.

GSK will handle the development, regulatory, commercialization activities, and costs of the drug, while Ionis will obtain license fees and milestone payments of up to $262 million, including a $25 million license fee.

The new vaccine will offer protection against the Nipah virus after a single dose.

Innovate UK has awarded a grant worth thousands to the Atelerix Consortium for the collaborative work with the Cell and Gene Therapy Catapult and Rexgenero on development of cell stabilization technology.

The new lab will be dedicated to improving materials and electronics using advanced imaging and surface analysis expertise.

The agreement gives Biotheus access to Alligator’s antibody, Alligator-Gold, for the creation of up to three bispecific molecules.

Marchesini Group’s Compact 12 electronic counter with HarleNIR vision system measures product and active ingredient when filling and capping bottles for tablets and capsules.
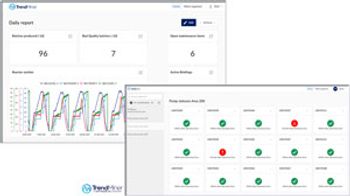
TrendMiner 2019.R3 displays a customized, actionable dashboard with a real-time view of the production process.

The company’s new training center in Greenville, NC, will use virtual and augmented reality to train employees on its sterile injectables manufacturing line, which is expected to cut training time in half.

Pfizer is investing $500 million for the construction of a state-of-the-art gene therapy manufacturing facility in Sanford, NC.

The Birtley, UK facility added cleanroom, manufacturing, and testing facilities for biopharma manufacturing single-use systems.

RINVOQ for the treatment of adults with moderately to severely active rheumatoid arthritis has been approved by FDA

ACG Engineering, which is a part of the ACG Group, has announced its acquisition of a German-based pharma processing equipment provider, Xertecs.