CDMOs strive for flexible, adaptable processes and fast tech transfer for manufacturing investigational drug products, including COVID-19 treatments and vaccines.

CDMOs strive for flexible, adaptable processes and fast tech transfer for manufacturing investigational drug products, including COVID-19 treatments and vaccines.

The Double Planetary Mixer from ROSS is suitable for high-precision mixing, granulation, and vacuum drying all in one.

ACG Group introduced the GT 1600 X.ONE, a line of integrated granulation equipment designed for high-volume batch production.

Pharmaceutical Technology spoke with Tania Pereira Chilima, Deputy CTO at Univercells Technologies about their technology and how it can be used in viral vector manufacturing, which is needed for production of some of the COVID-19 vaccine candidates.

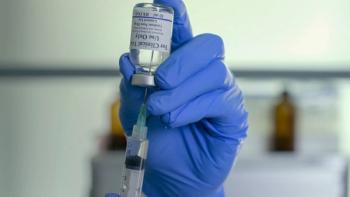
Moderna has filed for authorization for its COVID-19 vaccine candidate, which has shown high efficacy and safety in a Phase III trial.

The expansion will include new suites for the development and clinical manufacturing of drug product intermediates and drug products and cGMP suites for early-phase cGMP manufacture.

Originally announced in March 2020, the acquired facility will be Bora’s first North American manufacturing facility and will serve as its North American headquarters.

The added space will house a portion of its PVC, polyurethane, silicone, and additional plastic tubing products, as well as its AdvantaPure products.

The expansion is part of a new expansion initiative aimed at increasing flexibility, efficiency, and cost-effective production.

Wacker will support production of CureVac’s COVID-19 mRNA-based vaccine candidate at its biotech site in Amsterdam, with production scheduled to start in the first half of 2021.

The expansion will include a new 25,000-ft2 warehouse, a filling line for flexible plastic containers, a high-speed automated syringe fill line with the ability to fill up to 600 units per minute, and a high-speed automated visual inspection line.

The EC’s approval comes after positive results from seven Phase II and III randomized, active-controlled, multi-center studies.

A new facility near Frankfurt, Germany expands Avantor's geographic reach and supports the growing demand for scientific research and clinical trial sample storage.

The new super plant, being built in Incheon, South Korea, will have a total biopharmaceutical manufacturing capacity of 256,000 L.

The companies have formed a long-term manufacturing agreement to accelerate the global supply for Lilly’s COVID-19 antibody therapeutics.

The agreement follows a recent announcement that Sartorius plans to invest $100 million in Songdo, South Korea’s bio cluster.

The agreement will allow Roche to use CEVEC’s proprietary ELEVECTA technology, which is designed to enable fully scalable, high-performance AAV vector production in suspension bioprocesses.

The partners have established PathoQuest, Inc., a US subsidiary, and will construct an NGS-based testing lab at Charles River’s Wayne, PA, site.

An independent data safety monitoring board found that clinical trial data demonstrated that the vaccine candidate met its primary endpoint and demonstrated protection from COVID-19.

Following an EUA application from Pfizer and NioNTech, FDA outlines the process for committee and agency review.


Watson-Marlow Limited Ireland has revealed plans for a new ISO 14644-1 Class 7 cleanroom to be added to the company’s existing site in Cork, Ireland.

CordenPharma has announced the expansion of its non-GMP SPPS manufacturing capacity at its Centre of Excellence for Peptide Process Development and non-GMP manufacturing in Frankfurt, Germany.

Bone Therapeutics has reported the completion of Catalent’s acquisition of Skeletal Cell Therapy Support SA (SCTS)—Bone Therapeutics’ cell therapy manufacturing subsidiary.

Eisai has announced an expansion of its manufacturing operations at its Hatfield production plant in the United Kingdom to meet global demand.