
The overall market size of the Southeast Asia region totals $7 billion, with a projected compound annual growth rate of about 13% through 2010.

The overall market size of the Southeast Asia region totals $7 billion, with a projected compound annual growth rate of about 13% through 2010.

For once, casting originators against generic players might end up strengthening the industry across the board.

As the pace of product development accelerates, the approach to dissolution-method development must advance beyond a manual method and an assay. A natural progression of the method-development process must include the transfer of the manual method onto automated instrumentation.

Before formal cleaning validation programs were instituted, visual inspection was the primary means of determining equipment cleanliness. The use of visual inspection is still typically a component of a cleaning validation program and for routine inspections of cleaning effectiveness, but the use of visual inspection as a sole criterion for equipment cleanliness has not been successfully implemented as a valid approach for cleaning validation.

A hybrid system using paper and electronic pedigrees will be needed.

Partnerships launched 63 research projects that may translate into nine or 10 new drugs by 2010.
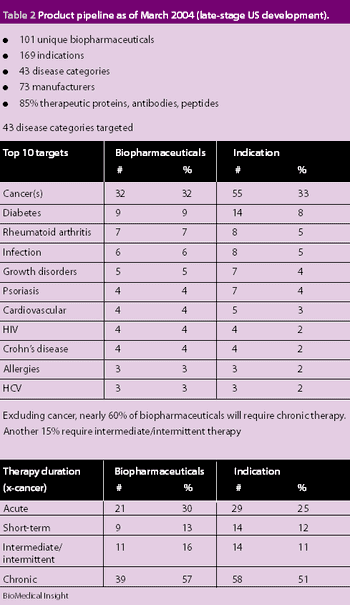
The worldwide market for biopharmaceuticals was estimated to be $50 billion in 2005. North America accounts for 60% in terms of revenue and R&D. Europe accounts for 20% and Japan 10%. It is also estimated that 400–500 biotech drugs are under clinical development for various disease conditions. Biopharmaceuticals are being developed to fight cancer, viral infections, diabetes, hepatitis and multiple sclerosis. The distinct families of biopharmaceuticals include
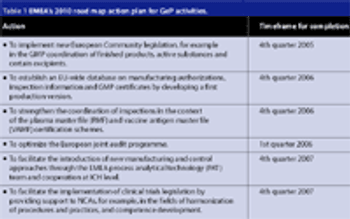
Since the formation of the European Union (EU) in 1993, each member state has brought along its own regulatory baggage, namely the standards and regulations that their companies are formally required to comply with. These standards and regulations still apply for any pharmaceutical products a native manufacturer decides to market within their homeland. When the same manufacturer markets its pharmaceutical products to consumers in other EU member states, the regulatory directives of the European Commission and the European Agency for the Evaluation of Medicinal Products (EMEA) apply as well.

RFID is viewed by many, including FDA, as a technology with strong potential for carrying the mass serialization data needed to track and trace product and to create pedigree records.

Process development is an important aspect of biopharmaceutical development.1,2 Through studies in the pharmaceutical industry, Pisano suggests that companies able to develop and implement new process technologies quickly and effectively have a competitive edge. In addition, the fact that the production cost of biopharmaceuticals could be up to 25% of sales value means that the failure to develop a viable process could result in uneconomic manufacturing routes and the inability to capture fully the value of the firm's discovery.3

There is currently no vaccination or cure for prion diseases and contamination of a parenteral or infusion pharmaceutical product could prove fatal.

With a finger on the pulse of an evolving market, the sales and marketing team can visually demonstrate trends and new market opportunities to the R&D team

Introducing lean manufacturing into the pharma sector.

Cardinal Health halted production, sales, repairs, and installations of its "Alaris Signature Edition Gold" infusion pump after the US Food and Drug Administration (Rockville, MD) seized approximately 1300 units last Friday. The seized infusion pumps (model numbers 7130, 7131, 7230, and 7231) have a "key bounce" defect that may cause overinfusion of medications by more than 10 times the intended infusion rate.

The generic drug company Mylan Laboratories, Inc. (Pittsburgh, PA) plans to acquire a controlling interest (71.5%) in the Indian pharmaceutical company Matrix Laboratories, Ltd (Hyderabad, India) for $736 million.

Merck KGaA (Darmstadt, Germany) plans to invest 190 million euros ($245 million) to build a biopharmaceutical plant in Darmstadt, Germany. Production is expected to begin in 2010.

Merck acquires Serono

Washington, DC (Aug. 14)?A new report issued by the Pharmaceutical Research and Manufacturers of America identifies 418 drugs and vaccines developed through biotechnology. All of the biotechnology medicines and vaccines are now in clinical trials or awaiting approval by the US Food and Drug Administration (Rockville, MD).

Teva Pharmaceutical Industries Ltd. announced that the company will cease production at its manufacturing facility in Cidra, Puerto Rico during the fourth quarter of 2006.

The International Organization for Standardization (ISO, www.iso.org) has formally issued and published standard ISO 4644-8:2006, Cleanrooms and associated controlled environments-Part 8: Classification of airborne molecular contamination. The document covers the classification of airborne molecular contamination (AMC) in cleanrooms and associated controlled environments in terms of airborne concentrations of specific chemical substances (individual, group, or category) and provides a protocol to include test methods, analysis, and time-weighted factors within the specification for classification.

ZymoGenetics (Seattle, WA) has sued Bristol-Myers Squibb (New York, NY) over alleged infringement of ZymoGentics's fusion-protein technology patents.

Akzo Nobel (Arnhem, Netherlands) is moving forward with plans to separate its pharmaceutical business into a separate company, Organon Biosciences

The Shenzhen government is planning to build a new national biopharmaceutical park in the Shenzhen Grand Industrial Zone.

Aegis Therapeutics (San Diego, CA) has launched a new formulation technology to increase the stability of proteins and peptides.

MSD Technology Singapore Pte Ltd., a wholly owned subsidiary of Merck & Co., Inc. (Whitehouse Station, NJ) dedicated an expansion of its production facilities in Singapore on July 28.