
A pneumatic cover lift for the Witte fluid bed dryer raises the cover for cleaning and inspection.

A pneumatic cover lift for the Witte fluid bed dryer raises the cover for cleaning and inspection.

The voluntary recall is due to blister packages containing the incorrect product.

The agency published guidance regarding OTC aspirin products that have cardiovascular-related images in their labels.

Supply Chain Wizard has developed a set of integrated digital solutions to improve supply chain functions.

CDMO Celonic acquired a biomanufacturing facility in Heidelberg, Germany from Glycotope.

Data governance is necessary for compliance with current regulatory expectations for data integrity in pharmaceutical R&D and manufacturing organizations.

Capsugel announced on Oct. 31, 2017 that it expanded clinical trial capabilities, as well as increased development and manufacturing capabilities for specialized drug products using liquid-filled hard capsule technology, at its Edinburgh facility in Scotland.

The companies will collaborate to develop and manufacture Grid’s lead therapeutic candidate for the treatment of solid tumors.

Experts from Colorcon share insights on how manufacturers can play a role in minimizing the risk of medication errors by ensuring that the medicines they develop are well differentiated, especially between dosages of the same product.

The shift toward continuous manufacturing among European pharmaceutical manufacturers has not been accompanied by a similar strong increase in the use of automation and sensor-based on-line monitoring.
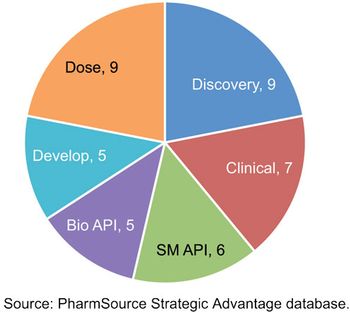
Recent acquisitions are creating CDMOs with scale that rivals global bio/pharma.

The revised Akers-Agalloco aseptic risk assessment and mitigation method includes two sub-methods that can be used independently for risk assessment.

Industry experts were honored for business, scientific, and social contributions.
In Part I of this article series, the regression control chart method for identifying out-of-trend data in pharmaceutical stability studies is investigated, and an improved approach is suggested.

The 300-gallon process vessel from Ross Charles can be customized according to specific user needs.

The K3-PH-ML-D4-QT35 feeder from Coperion K-Tron is suited for multi-feeder clustering in a variety of continuous processes.
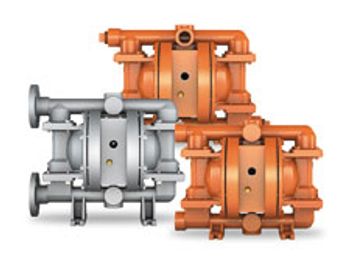
Wilden's air-operated, double-diaphragm pumps feature bolted product containment, self-priming and dry-run capabilities, and are direct replacements for existing clamped and bolted pumps from the company.

Eli Lilly was awarded the 2017 FOYA Overall Winner for its continuous direct compression manufacturing installation projects.

The Almac Pod, a temperature-controlled shipping solution service for biologics and other temperature-sensitive products, is compliant with good distribution practices and comes in three models.

Despite GxP and data-management challenges, pharma is moving toward new models for clinical trial logistics.

FDA announced a public workshop to explore strategies for addressing the crisis of opioid addiction through innovations in packaging, storage, and disposal.

A mutual recognition agreement to accept inspection data from eight EU regulatory authorities may reduce inspection burdens at global drug facilities.

The biotechnology company has entered a contract with the United States Army to develop custom recombinant spider silk for protective textile applications.

Preventative maintenance of heat transfer fluid systems is important for reducing downtime in pharmaceutical manufacturing.

The company has passed inspection by the UK Medicines and Healthcare products Regulatory Agency, and is now licensed to develop, manufacture, and pack non-potent and potent solid oral drug products at its site in Loughborough, England.