
Celegance’s Dossplorer can import product registration dossiers and ready regulatory submissions for electronic common technical documents compliance.

Celegance’s Dossplorer can import product registration dossiers and ready regulatory submissions for electronic common technical documents compliance.

Bio-Rad Laboratories’ Pioneer Antibody Discovery Platform is an antibody discovery service specifically designed to develop best-in-class biologic candidates.

Webinar Date/Time: Thursday January 19, 2023 at 11am EST | 10am CST | 8am PST

The complaint states the company introduced adulterated drugs into interstate commerce that were manufactured, processed, packed, or held under conditions that defy current good manufacturing practice (CGMP) requirements.

In this episode of the Drug Solutions Podcast, Meg Rivers discusses outsourcing strategies in biopharma with Jeff Henderson, key account manager of Vetter.
Some manufacturers are developing smaller, more mobile drug manufacturing processes for point-of-use patient care.

Emmes’ new facility is designed to support clients conducting cell and gene therapy research worldwide.

Thermo Fisher Scientific’s new facility in Hangzhou, China, is designed to boost biologics and sterile development and manufacturing capabilities in the Asia-Pacific region.

mRNA-1273.222 has also received FDA EUA for children and adolescents between six and 17 years of age and for adults over the age of 18 years of age.

The R&D analytical solutions will consist of analytical lab services that use USP’s in-house scientific expertise and state-of-the-art facilities at the USP Advanced Manufacturing Technology Lab in Richmond and the USP headquarters facility in Rockville, Md.

The most safe and effective therapies demand the highest data quality.

In this episode of the Drug Solutions Podcast, Chris Spivey interviews executives at Shabas Solutions LLC, who ran the overseas QMM pilot project.

Advances in contamination control are being to mitigate the presence of pollutants in drug products.

One can improve method precision and productivity by replacing one step in sample preparation with an automated approach.

Advancing digital transformation can significantly reduce R&D costs and shorten drug discovery timelines.

Overall industry growth conceals growing numbers of smaller, faster-paced, adaptable corporate structures.

Unique dosage forms, personalized medicine, and flexible manufacturing are possible with 3DP.

mRNA may not be a train we necessarily all need to get onboard immediately, but we should know where the central train station is located, and what it connects us to.

The ROSS Vertical Blender enables mixing of friable solids.

Steraline’s VFCM100 guarantees sterility throughout the aseptic filling process via a double-wall isolator.

ACP Systems’ quattroClean snow jet technology uses liquid, climate-neutral CO2 for cleanroom cleaning.
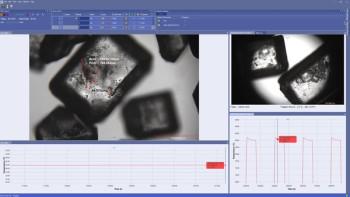
Linkam Scientific Instruments’ NEXUS software enables holistic environmental controls in experiments.

Catalent has completed the expansion of its clinical supply facility located in Shanghai, China.

The agreement will see Immutep and Merck KGaA, Darmstadt, Germany jointly fund the INSIGHT-005 study.

Webinar Date/Time: Thursday, December 8, 2022 at 11am EST | 10am CST | 8 am PST