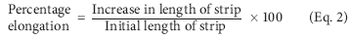A company's contamination-control plan is an important document designed to formalize the rationale, methods, and validation of contamination-control procedures in a manufacturing facility. The author describes the role of bioburden in the contamination-control plan.