
Advanced analytical tools generate more data in today’s labs than ever before.

Advanced analytical tools generate more data in today’s labs than ever before.

Interpreting data and understanding the various components of biologic drug substance testing is an important skillset to know as a lab personnel.

Brandon McNaughton, founder and CEO of Akadeum Life Sciences, discusses his start-up’s unique microbubble separation technology.

Stuart Malcolm, head of Standards, Efficiency, and Automation at Veramed, speaks on how advancing technologies are shaping clinical trials.

The collaboration between Parexel and Partex is designed to leverage artificial intelligence-powered solutions to accelerate drug discovery and development.

Caroline Phares, global head of Health and Life Sciences at Domino Data Lab, speaks on integrating data processes into pharma operations.

Cytomos will use the £4 million in funding to develop its dielectric spectroscopy technology.

Waters XBridge Premier GTx BEH size exclusion chromatography (SEC) columns are designed to improve analysis while lowering the cost of gene therapies.

Laboratories are focusing on more automated processes while also trying to maintain an efficient, safe, and personalized experience with each analytical test.
The industry is taking steps to automate the final product inspection process for complex therapeutics.

Interoperability difficulties resulting from discrepancies in preferred programming languages can be resolved via statistical computing environments.

Environmental monitoring data can help keep sterile environments sterile.

Kelly Doering, senior director at Aspen Technology, unpacks various strategies for holistic data integration into pharma operations.

Thermo Fisher Scientific’s new tumoroid culture medium kit is designed to help cancer researchers better model the disease.

Eurofins Genomics Blue Heron’s novel mRNA synthesis service is designed for customizable applications.

When evaluating a drug’s risk assessment for elemental impurities, one must consider all aspects of its lifecycle.

When evaluating a drug’s risk assessment for elemental impurities, one must consider all aspects of its lifecycle.
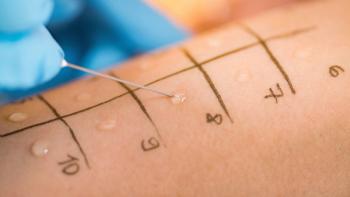
FDA’s new guidance on in-vitro permeation test studies published in October 2022 gives technical and statistical requirements for conducting these tests to compare topical generic drugs with their reference products.

Data from environmental monitoring can assist in keeping sterile environments sterile.

Automation can be balanced with operator oversight.

The Segmentation by Exogenous Perfusion system uses a cell’s location in the tumor to find differences in gene activity.

Sandbox AQ’s molecular simulation division is collaborating with companies like AstraZeneca and Sanofi to develop novel treatments for various disorders.

Charles River’s off-the-shelf lentiviral vector packaging plasmids are intended for use with cell and gene therapies.

Sartorius and Waters will collaborate to develop integrated analytical solutions for downstream biomanufacturing.

A holistic data integrity approach can help one avoid missteps in data integrity.