
The body’s over-editing of synaptic interactions is recognized as a probable cause of schizophrenia in a landmark study.

The body’s over-editing of synaptic interactions is recognized as a probable cause of schizophrenia in a landmark study.

Planned expansion at Onyx Scientific add laboratories, GMP suites, and storage space.

Andrew Badrot, founder and CEO of Centroflora CMS, discussed the acquisition and technical transfer of Boehringer Ingelheim’s (BI) non-captive phytochemical API portfolio with Pharmaceutical Technology.

The denial marks a setback for Amgen, who’s Humira biosimilar ABP 501 is the first submitted to FDA for adalimumab.

FDA issued a warning letter to Zhejiang Hisun Pharmaceutical Co., Ltd., as a result of inspections that took place on March 2–7, 2015 at the Taizhou City, Zhejiang Province, API manufacturing facility.

The collaboration will focus on development of novel molecules using SMARTag technology to couple different therapeutic modalities.

More “me-betters” and more focused breakthroughs could enhance new drug development.
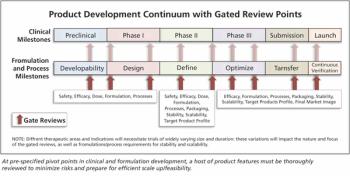
Appropriate use of sound science applied at critical junctures will improve efficiency in the high-wire act of drug development.

The author discusses the collection and evaluation of data part of FDA’s definition of process validation.

DAVID LEAHY/GETTY IMAGESThe pharmaceutical industry has an important role to play in implementing solutions to global envi
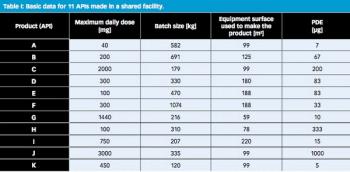
The 10-ppm criterion for the acceptable concentration of potential API in cleaning validation to minimize cross contamination into next product has been employed for many years. This article describes why the 10-ppm criterion, which was established based on analytical limitations and estimates of acceptability, is no longer necessary and why a risk-based approach should be universally adopted.
Nanoscale catalysts and engineered nanoparticles may have a big impact on small-molecule pharmaceuticals.

Amid corporate restructurings, regulatory initiatives, and aging R&D assets, will drug development accelerate or stall in 2016?

FDA approved 45 novel new drugs in 2015, the highest number of approvals since 1996 and second-highest ever.

Leading nations are backing moves to strengthen WHO’s central role in international health security following the Ebola crisis, which sparked criticisms on the organization’s ability to address a pandemic outbreak.

Liquid formulations in hard-shell capsules or softgels are becoming a popular option for HPAPIs because of advantages such as improved safety and lower risk of potential exposure and product cross contamination.

Synthetic biology promises to drive tomorrow’s therapies, while continuous processing is already being used in some new drugs.

BASF and Sumitomo Chemical explore in vitro system for chemical safety evaluation.

Mass Spec Lab, a privately owned analytical company, announced its official launch on Dec. 28, 2015 in Southern California.

Boehringer Ingelheim announced it will establish a new biopharmaceutical production facility in Vienna.

The aim of the collaboration is to advance the use of Cellectar’s phospholipid drug conjugate platform for targeted delivery of a selection of Pierre Fabre’s cytotoxics.

Researchers from Oregon State University develop a new three-drug delivery system for cancer treatment.

AstraZeneca partners with the Wallenberg Center for Protein Research to conduct studies on the Secretome.

Assembly Biosciences reported a successful clinical study validating the use of the Gemicel technology platform for the oral delivery of biologic medications.

The agency publishes draft guidance on best practices for communication between FDA and IND sponsors during drug development.