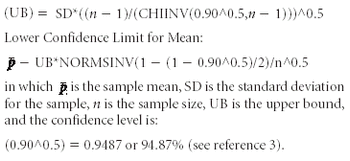
Using the Bergum Method and the MS Excel software program, the author determines the probability of passing the USP dissolution test.

Using the Bergum Method and the MS Excel software program, the author determines the probability of passing the USP dissolution test.

SEC Loosens Revenue-Recognition Rules for Vaccine Stockpile Participants

NIAID Boosts Vaccine Innovation but Draws Controversy
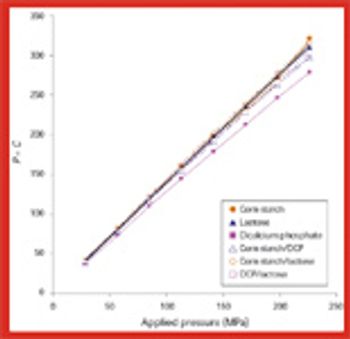
The survival of Bacillus subtilis spores in dicalcium phosphate, lactose, and corn starch and in their binary mixtures depends on the compressional properties of these materials and on parameters involved during the tableting process, including compression speed.
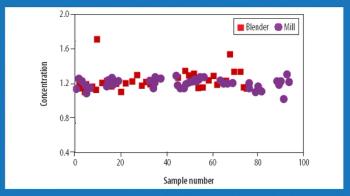
Almost all pharmaceutical manufacturing processes require handling and processing cohesive powders. The application of sufficient shear (i.e., the total deformation that the bulk of granular material undergoes under applied shear stress) is an essential factor in such processes. Sufficient shear is required to mill and de-lump materials, achieve sufficient flow, and homogenize cohesive ingredients. Shear mixing plays a critical role in the blending of dry powders, particularly for those that contain a minor cohesive component such as a solid lubricant or a drug. This mechanism is necessary to achieve a satisfactory homogeneity and disintegrate possible agglomerates. Excessive shear can be disadvantageous, however, and can lead to electrostatic buildup, attrition, and overlubrication.
Solid oral drug products are one of the oldest of all manufactured dosage forms (1). Today, the development of an appropriate formulation of drug and excipients and of an effective manufacturing process to create a tablet or capsule is slowly transforming from a practice of applied art to one of applied science. The US Food and Drug Administration supports this change by expecting sponsors of new drug applications to understand, describe, and control materials and processes as well as the risks associated with drug product manufacturing (2). These steps will ensure the consistent production of products that meet their specifications and remain safe and effective during their shelf life.

GSK Study Questions Bioequivalency of Generic Cold Sore Creams

Adjuvant for HPV Vaccine Enhances Immune Response Levels

Chiron Looks to Flu Vaccine Cell Culture; FDA Gears Up

Control Development's (South Bend, IN, www.controldevelopment.com) blend uniformity and dryer monitor consists of a NIR spectrometer, a sampling head, computer, and software.

Generally, tablet and capsule film coatings are applied as aqueous or organic-based polymer solutions or dispersions, graduate student Sagarika Bose (University of Connecticut) explained during her Tuesday AAPS Graduate Student Symposium presentation, "Development and Evaluation of Solventless Photocurable Pharmaceutical Film Coating." However, organic film coatings can be flammable, toxic, and must comply with strict environmental regulations. Aqueous film coating can lead to the degradation of certain drugs by heat and water.

When it comes to developing a robust lyophilization process, formulators can "pay now or pay later," says Jeff Schwegman, PhD, founder and chief scientific officer for BioConvergence. Because 30% of new drugs in clinical trials are biotech-based therapeutics (compared with 7% 10 years ago), more than ever, the US Food and Drug Administration is paying close attention to lyophilization data and questioning pharmaceutical companies about their development cycles, especially cycle development transfer, shelf-temperature mapping, dryer-to-dryer comparison studies, formulation time, process validation, and cycle deviation. Consequently, this is pushing formulators to optimize formulation variables, conduct additional testing during early-stage development, and understanding critical process parameters, equipment qualifications, and manufacturing conditions that can influence formulation behavior at a large scale. Not taking the time or effort to achieve these goals during early development could lead to redundancies in formulation work - a reality observed too often in today's practices.
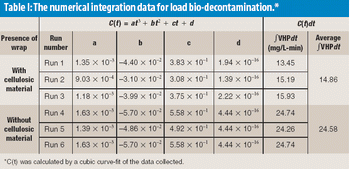
The authors focus on the effects of cellulosic materials during the performance qualification validation of a transfer barrier isolator used for the purpose of sterility testing.
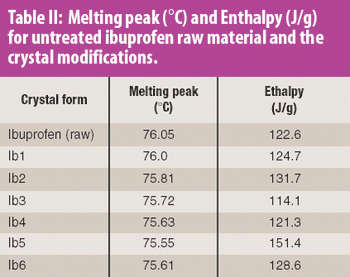
The common crystal form of ibuprofen was changed to optimize processing and manufacturing properties. Six modified crystal forms were prepared and assessed for dissolution, morphology, particle size, density, thermal characteristics, powder x-ray diffractometry, flow properties, and tabletability.

Biomanufacturing managers believe the current lack of adequately trained personnel is one of the most serious problems facing the biomanufacturing industry.

Industry, FDA, and World Health Agencies Boost Pandemic Preparedness

Roche Plans New US Manufacturing Facility for Tamiflu while Cipla Prepares to Market Generic

Collaboration Targets Single-Shot Japanese Encephalitis Vaccine
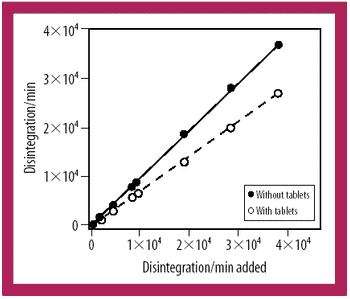
A radiotracer technique is a simple, fast, and sensitive technique for analyzing the integrity of clinical supply packages to water.

This review article discusses orally disintegrating tablets and their manufacturing technologies, development issues, and future trends.
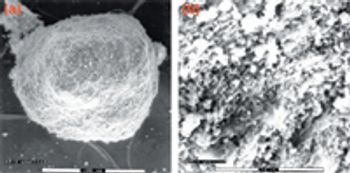
Although agitation improves drying efficiency and ensures uniformity of the final dry material, it can also affect the physical properties of the product as it dries. This study evaluates the effect of scale up and equipment selection on an active ingredient undergoing granulation during the drying process.

A fleet of pharmaceutical manufacturers have green lighted new bar coding and e-pedigree pilots using electronic barcodes. But is Industry ready for full-scale RFID?

Pharmaceutical science and technology innovation

Genecor Wins Vaccine-Development Contract

FDA Proposes Question-Based-Review System for Generics