The identification of an increasing number of drug targets coupled with advances in manufacturing technology could lead to many more blockbuster therapeutic antibodies.

The identification of an increasing number of drug targets coupled with advances in manufacturing technology could lead to many more blockbuster therapeutic antibodies.
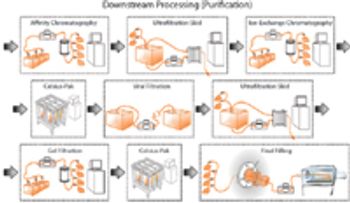
The authors discuss current and future disposable technologies and outline the validation and qualification steps that would be required for a possible disposable process stream.

The authors review the role of automation in aseptic processing and describe their experience in implementing advanced technologies, including the use of isolators and robotics.

Follow-on biologics or biosimilars offer a niche growth market for the pharmaceutical industry. As the process for establishing a regulatory pathway for biosimilars is debated, companies, including Big Pharma, are positioning themselves to gain this piece of the pharmaceutical pie.
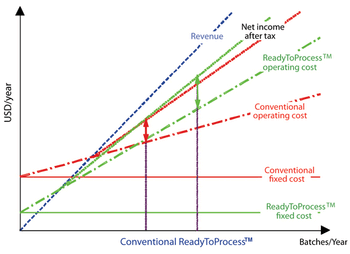
The number of biotechnology-based human therapeutic products in the late-stage pipeline along with the average cost to commercialize a biotech product has been steadily increasing with time. In addition, the biotech industry is facing unprecedented challenges of a sagging global economy and rising regulatory expectations. Companies have to continue to evolve their approaches to be more efficient with respect to time, resources and cost. This article describes some of the technologies that can help optimize time and cost of biopharmaecutical manufacturing.

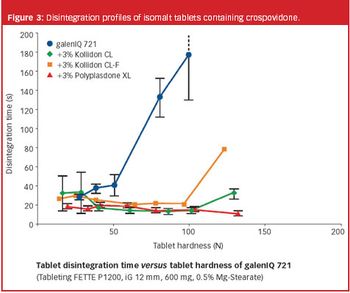
The authors examine common superdisintegrants (i.e., crospovidone Type A, crospovidone Type B, croscarmellose sodium, and sodium starch glycolate) with a set of poorly soluble drug actives and evaluate in vitro drug dissolution.

A bipartisan bill that would establish a regulatory pathway for the approval of biosimilars was introduced into the US House of Representatives last week.

The spotlight on the biopharmaceutical industry is intensifying, as recently evidenced by Pfizer's (New York) ongoing acquisition of Wyeth (Madison, NJ), which was initiated partly to reduce the former's dependence on small-molecule drugs.
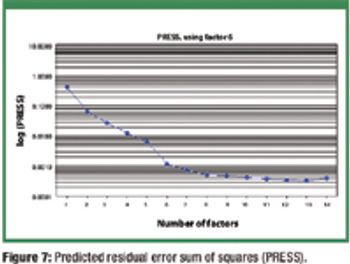
The authors extend the range of a near-infrared calibration model for tablet assay using production 'seed' spectra and synthetic spectra generated from placebos and 'pure' active pharmaceutical ingredient spectra.

Michiel Ultee talks about the challenges involved in transferring technology to a CMO and why communication is so important.

The productivity of the pharma industry has been declining for several years. Could biotechnology reinvigorate drug development?
PAT guidance has been available from FDA for more than 4 years, but there have been no apparent breakthroughs in large-scale upstream production. Will companies consider using on?line chromatography to change this?

After two years of hearings, more than 5000 pages of expert testimonies, and 939 medical articles, a special federal court ruled that there was little, if any, evidence to support the claim that substances in the measles, mumps, and rubella vaccine (including the use of thimerosal) had led to the autism of three children.

The US Food and Drug Administration withdrew this week its direct final rule on Reporting Information About Authorized Generic Drugs.
During the past years, there has been increasing demand for fast dissolving disintegrating tablets (FDDTs), such as orally disintegrating tablets (ODTs) and sublinguals.

A discussion of the basic principles of immunity and the nature of antibodies.

The US Department of Health and Human Services's Biomedical Advanced Research and Development Authority has awarded Novartis a contract valued up to $486 million over eight years to support the design, construction, validation, and licensing of a US cell-based influenza vaccine manufacturing facility in Holly Springs, North Carolina.

Outsourcing sterile manufacturing involves an integrated approach in product life-cycle management.
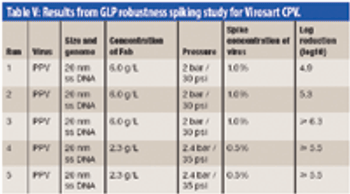
The authors examine the challenges of integrating a large-scale chromatography and nanofiltration process for purification of a polyclonal antibody.

The biotechnology sector is occasionally described as a rainbow, with each sub sector having its own colour. But what do the different colours of biotechnology have to offer the pharmaceutical industry?
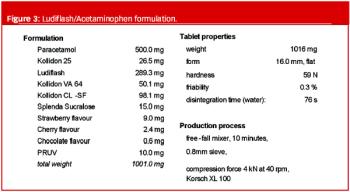
A new excipient for orally disintegrating tablets not only imparts superior tablet characteristics, but has the added advantage of allowing users to maintain full control over their formulations, manufacturing processes and intellectual property.

A new year, all things being equal, is a time of looking to the future with positive intent and traditional bonhomie. At the very least, it suggests new beginnings. As we enter 2009, however, the world is moving into a major recession and things are far from equal.

Reducing ecological footprint and achieving more sustainable production of pharmaceuticals could help create a better future.

A US Food and Drug Administration guidance issued Tuesday provides new recommendations to applicants who wish to designate proposed products as orally disintegrating tablets (ODTs).

At its annual business briefing held last week, Merck & Co. outlined its short- and long-term strategy for growth. Its strategy is focused on increased penetration in emerging markets, the establishment of a business for developing follow-on biologics or biosimilars, and a new commercial model for product life-cycle management.