
Pharmaceutical Technology® spoke with Will Gordon, senior vice president of Product Management, ArisGlobal, about the impact of artificial intelligence and machine learning on the bio/pharmaceutical industry.

Pharmaceutical Technology® spoke with Will Gordon, senior vice president of Product Management, ArisGlobal, about the impact of artificial intelligence and machine learning on the bio/pharmaceutical industry.

The CytoFLEX mosaic Spectral Detection Module offers up to 88 channels for detection.

Three of the five are biosimilars, and seven additional medicines received recommendations for indication extensions.

Sherwin-Williams’ advanced coating systems are designed to ensure safety, sterility, and efficiency in pharma manufacturing environments.

Winners are selected entirely through industry voting, with those honored representing recognition by their peers.
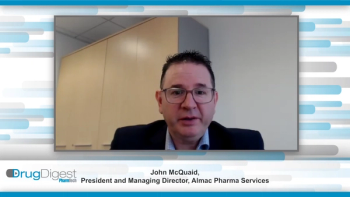
In this exclusive Drug Digest video interview John McQuaid from Almac Pharma Services and Sridevi Khambhampaty from Shilpa Biologics delve into the evolution of the bio/pharma outsourcing market and look at how service-providers’ strategies have adapted to meet demand.

In the latest of a series of warning letters to India-based API manufacturers, FDA issued a warning letter to Aspen for deviations in CGMP in the production of APIs.

The Biotechnology Innovation Organization’s new membership survey said that 90% of US biotech companies rely on imported components for at least half of their FDA-approved products.

With a completed €40 million (US$43 million) investment in Slovenia, Novartis has opened its first specialized viral vector production facility in Europe, following earlier significant investments in R&D that has led to the growth of Slovenia’s workforce.

In response to a presidential Executive Order, the Department of Health and Human Services is planning to reduce its workforce, including cutting FDA staff by 3500 full-time employees, and reorganize some key departments.

The company’s state-of-the-art sterile drug product manufacturing site in Saint-Beauzire, France, opened in February 2023.

The contract will support R&D of high-quality APIs for substance use disorders and mental health conditions, starting with synthesis and scale-up of psilocybin, which is derived from mushrooms.

Under the agreement, AstraZeneca will use Alteogen’s proprietary hyaluronidase platform technology to develop and commercialize subcutaneous formulations of multiple oncology assets in its portfolio.

Fabhalta (iptacopan) received a positive opinion for treatment of C3G from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) and has been approved twice before by FDA.

The EU medicines agencies’ network strategy, EMANS, is an update of the five-year strategy previously developed to cover the period 2021 to 2025.

With financing led by Blue Owl Capital, Latigo will use the funds to further develop non-opioid pain treatments as well as develop other candidates in its pipeline.

PharmTech Group chatted with Markus Laubscher of Orbia during DCAT Week 2025, covering the topics of sustainability, regulatory hurdles, next-generation innovation, and the DCAT Week experience.

Upgraded and dynamic valve platforms as well as advanced sensing will be some of the components of ITT’s exhibit.

The Flexmag 4050C flowmeter has a biocompatible, disposable flow tube that Krohne says has been specifically developed for single-use applications such as filtration processes or chromatography.

Dirk Margosch, Vice President Visual Inspection, Assembly & Secondary Packaging at Vetter Pharma-Fertigung GmbH & Co KG, provides advice on final product inspection for small batch sizes. He also discusses how to maintain cold chain during visual inspection.
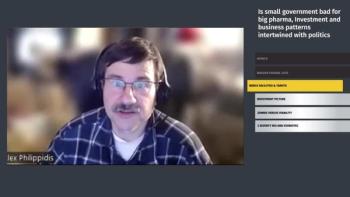
Neil Hunter, Alex Philippidis, Joanna Sadowska, PhD, EMBA, and Carl Schoellhammer, PhD go behind the headlines to discuss the impact of federal budget cuts on future pharma investments, reshoring efforts, and biotech buyouts.

In an interview with Pharmaceutical Technology®, Michelle Bridenbaker from Unbiased Science discusses her thoughts on the key industry from 2024 and those she anticipates will impact the industry in 2025 and beyond.

Roquette Pharma Solutions' Christine Mya-San, global account manager, highlights the most significant pharma ingredients at play over the past year and discusses future ingredient innovation.

The company is highlighting its commitment to next-generation glass manufacturing in efforts to strengthen the pharmaceutical supply chain.
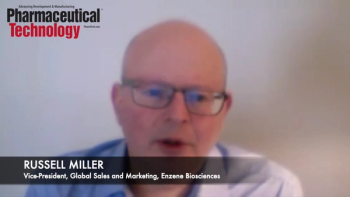
Russell Miller, vice-president of Global Sales & Marketing at Enzene, explains that continuous bioprocessing is poised to change biologics manufacturing moving forward.
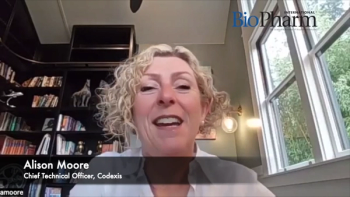
PharmTech Group spoke with Alison Moore, chief technical officer, Codexis to find out the latest about the RNA therapy market and to get perspective on how enzymatic RNA synthesis is used to synthesize RNA molecules.
Pharmaceutical Technology® sits down with Henny Zijlstra from Adragos Pharma to chat about the trends affecting the outsourcing market, various strategies being employed by service providers, and the value of end-to-end services.

PharmTech Group sat down with Anil Kane of Thermo Fisher Scientific live on site at DCAT Week 2025, to talk about technologies and digital solutions companies are using to meet demand across the bio/pharma industry.

In an interview with Pharmaceutical Technology®, Tore Bergsteiner from MAIN5 details his predictions for how the mega trends will shape the bio/pharma industry in 2025 and beyond.

PharmTech Group interviewed Lee Karras of Noramco live on site at DCAT Week 2025, shortly after the company announced a $25 million investment in sterile injectable manufacturing capabilities at its Halo Pharma facility in New Jersey.