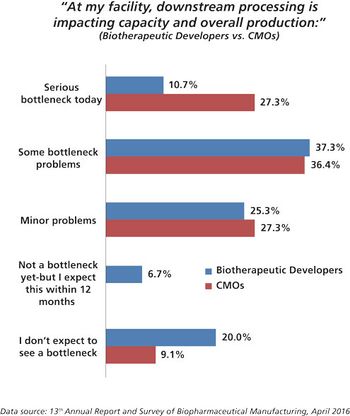
CMOs are working hard to improve performance by investigating new technologies for filtration and purification.

CMOs are working hard to improve performance by investigating new technologies for filtration and purification.

CMOs that offer an innovative service-oriented model will dominate the industry.

Stability testing programs should provide drug owners the information needed to establish the proper handling, shipping, and shelf-life recommendations for drug products.

Bio/pharmaceutical contract service provides continue to invest in development, facility upgrades, technological advancement, and mergers and acquisitions.

The company plans to purchase and develop an 18-acre site in Des Plaines, Illinois.

The company expanded its services to include oligonucleotide API development and manufacturing and received approval for its Caponago manufacturing facility.

A RoTab tablet press can product up to 42,000 tablets per hour for Juniper Pharma Services.

Uncertainty about the demand for a biologic medication can be partly mollified with some well-planned capacity outsourcing, contends a new report by ORC International sponsored by Patheon.

Industry experts discuss common considerations and recent technological advancements in blow-fill-seal technology.

The company expanded its topicals capacity with an investment in the Becomix RW30 model homogenizer.

PharmTech sat down with an intellectual property lawyer to examine how companies are protected when they engage in activities where sharing of trade secrets must occur.

At DCAT's annual meeting, Bill Downey, president of the market research firm High Tech Business Decisions, summarized results from its latest survey on biopharmaceutical outsourcing.

Collaboration will provide for unified development and manufacture of antibody drug conjugates.

Hovione will operate a commercial-scale continuous manufacturing facility in New Jersey as part of an agreement with Vertex Pharmaceuticals.

As specialty API outsourcing grows, manufacturers and contract development and manufacturing organizations are investing for the long haul.

PharmSource report addresses how the opportunity for antibody drug conjugates measures up to the bio/pharma industry’s expectations.

Sustainable harvesting combined with CMO expertise helped Centroflora CMS ensure supply continuity after it acquired Boehringer Ingelheim's non-captive API phytochemicals portfolio.
For successful partnerships, it’s important to take a long-term view, focus on simple designs, and address potential payer concerns up front.

Capsugel adds clinical trial and commercial manufacturing, as well as particle engineering services with two acquisitions.

Ash Stevens has received FDA’s approval to manufacture Takeda’s multiple myeloma drug, ixazomib, at its facility in Riverview, Michigan.

The University of Sheffield has appointed Cobra Biologics to advance novel fusion protein technology into Phase 1 clinical trials.

Expansions at Catalent’s Kansas City, MO, and Madison, WI facilities made in response to industry demand.

Operations at Catalent’s Beinheim, France, softgel facility were suspended following suspected deliberate action to misplace capsules.

Industry veteran Mark Kontny joins board of directors at Grand River Aseptic Manufacturing.
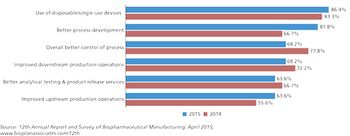
Better process development is creating industry benchmarks for bioprocessing.