
Pharmaceutical Technology Europe will be exhibiting at CPhI Worldwide at Stand 3G98.

Pharmaceutical Technology Europe will be exhibiting at CPhI Worldwide at Stand 3G98.

he guidance addresses the good manufacturing practice for managing quality in APIs.

Excipients play a crucial role in the manufacturing of solid-dosage forms and the performance of the finished drug product.

Researchers develop catalysts that mediate complex transformations under conditions appropriate for commercial manufacture.

The current approach of classifying process parameters and quality attributes as critical or non-critical is too simplistic to adequately reflect current science and risk-based approaches to product quality.

BASF’s new production facility in Shanghai will produce PVP K30 powder, a polymer used as a base for pharma excipients.

Dow and Colorcon extend and broaden the scope of the Controlled Release Alliance.

Clinical biotechnology company Moderna Therapeutics will build an integrated clinical manufacturing facility for mRNA production in Norwood, Massachusetts.

The certification follows a successful inspection by the MHRA, with no critical or major observations. The site is now ready to start production.

Saneca Pharma is making significant investment in its API capabilities to support client demand for smaller batch sizes and streamlined scale-up.

Researchers at GlaxoSmithKline report a greener and lower-cost route to chiral fluorolactams that is suitable for scale up.

Sound process understanding and having effective controls in place are crucial in ensuring that consistent product quality is obtained during API manufacturing.

IPEC consultant Irwin Silverstein discusses third-party audits and regulatory compliance issues facing suppliers and their customers.

The platform combines an expression system with equipment and process controls to enable rapid development and scale-up of robust titers.

API and drug product manufacturer changes name to align with parent company.

Dalton Pharma Services completed an expansion in sterile filling and API manufacturing at its cGMP facility in Toronto, Canada.

CDMO Alcami adds HPAPI capacity and cryogenic capabilities to its Wisconsin facility.

Irvine Scientific’s new product range includes chemically-defined, serum-free media, to increase productivity of viral vectors and recombinant proteins in suspension cultures.

Agilent Technologies announces plans to build a new oligo manufacturing facility in Colorado that will double current capacity.

Jacobs Engineering Group was awarded a contract to provide engineering services and procurement for Alnylam Pharmaceuticals’ new manufacturing facility in Norton, Massachusetts.

Recent research of efficient chiral synthesis technologies reveals potential uses in API manufacturing.

Enzymatic catalysis offers pharma manufacturers a way to implement the Principles of Green Chemistry.
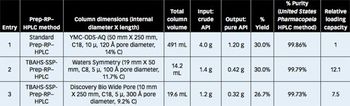
This paper describes a unique Prep-rP-HPLC technique that uses a C-18/C-8 derivatized silica coated with a hydrophobic quaternary ammonium salt or quaternary phosphonium salt that acts as an additional/surrogate stationary phase (AsP/ssP).

Multiple methods are required for detecting and removing protein impurities.

The therapeutic candidate AZD8601is an investigational mRNA-based therapy that will be tested for its ability to regulate the protein that influences vascular tissue growth.