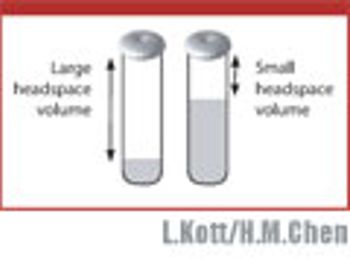
In this case study of amines, the authors discuss several parameters to be considered in developing a headspace GC method.

In this case study of amines, the authors discuss several parameters to be considered in developing a headspace GC method.

The growth of Brazil's generic-drug market is on a fast track, but what are the projections for the sector's future?

A Pharma Business Imperative.

When vessels, seals, and cooling units go haywire, operators must get in the mix.

The author proposes techniques, based on Six Sigma methods, for monitoring such processes to discover their airflow patterns and reduce opportunities for spillage.This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

Sample preparation before analysis is just as important as the analytical technique because poor sample preparation alters or skews the analytical result.

The needs of the pharmaceutical industry for powder testing technologies are changing, primarily for two reasons. The first is that global economics, and the economics and competitiveness of the pharmaceutical industry itself, are driving manufacturers towards achieving greater efficiency.

Testing the dissolution rates of a pharmaceutical formulation (also known as in vitro availability) aids drug quality control and is a compulsory requirement of the British, European and US pharmacopoeias.

Bioanalysis has migrated from a very general vocation to one that requires more specialisation, particularly in GLP applications.

Why spray drying processes offer a range of particle engineering possibilities.

Liquid chromatography-mass spectrometry (LC-MS) is a highly sensitive and specific analytical chemistry technique that is very commonly used in pharmacokinetic studies of new chemical entities.

The author assesses the power and limitations of NIR chemical imaging and its future.

Bioanalysis has migrated from a very general vocation to one that requires more specialization, particularly in GLP applications.

X-ray powder diffraction (XRPD) is a key analytical technique in the pharmaceutical industry, providing direct information about the crystalline and amorphous components in pharmaceutical formulations in a non-destructive manner and without laborious sample preparation.

NIR chemical imaging is based on the same principles as NIR spectroscopy, but uses a focal plane array detector in place of a single detector element to capture the spectral signature at tens of thousands of spatially resolved positions simultaneously.

Having been directly involved in the evolution of dissolution testing since its introduction in the early 1970s and variously representing Hanson, Van Kel, Sotax and Erweka prior to developing our own range of dissolution testers in 2002, I would have to say that advances in dissolution testing technology have been one of the biggest breakthroughs in solid dosage form testing over the last decade.

Charles River Acquires WuXi AppTec; Amgen Appoints President and COO; and More.

FDA Issues Warning Letters to Astellas, GSK, And Novartis; Sandoz Acquires Oriel Therapeutics; And More.

Cephalon Completes Acquisition of Mepha; Xcellerex Appoints President and CEO; and More.

The US Pharmacopeial Convention welcomed Minghao Zhou of China's Zhejiang Provincial Institute for Food and Drug Control.

Eli Lily Wins Court Battle; Genzyme Appoints COO; And More.

The Agilent 2100 Bioanalyzer plus the Agilent High Sensitivity Protein 250 Kit automatically detects and quantifies protein impurities down to 0.05% ? meeting ICH guideline Q3B(R2)!

In Part I of this article, which appeard in the March 2010 issue, the authors describe their approach for constructing form spaces for carbamazepine, cimetidine, and phenylbutazone by initial solvent screening to evaluate the feasibility of spherical crystallization. Part II of this article discusses their findings.

As the industry continues to evolve, do we know where we're headed?

Laboratory personnel share interesting tales as well as stories of unexpected tails.