The author reviews test methods for microbiological cleaning processes and suggests ways to improve microbial bioburden method suitability studies.

The author reviews test methods for microbiological cleaning processes and suggests ways to improve microbial bioburden method suitability studies.

Industry's focus on cost cutting has led to a dangerous gap in training and knowledge.

The authors discuss the statistical tools used in experimental planning and stategy and how to evaluate the resulting design space and its graphical representation.

Editors' picks of pharmaceutical science and technology innovations.

Merck and Sinopharm Sign Agreement; Agilent Appoints CFO; And More.

Pfizer Ends Second Tanezumab Clinical Program; Catalent VP Joins USP Panel; And More.

The US Food and Drug Administration has joined the Tox21 collaboration, which aims to develop ways to more effectively predict how chemicals will affect the body and environment.

The pharmaceutical industry?s increasing interest in inhaled drugs has prompted several researchers to propose standard dissolution-testing methods for these products.

Xcelience and Penn Form Joint Venture; Almac Appoints QA Director; And More.

Eli Lilly to Acquire Alnara Pharmaceuticals; Exelixis CEO Leaves for Biogen Idec; And More.
Nanotechnology is an important area of drug and biomedical research, and advancing nano-analysis is crucial for its further development.

A history of the selection of the widely used significance level leaves much to be desired.
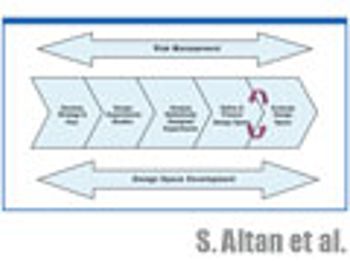
The authors discuss the statistical tools used in experimental planning and strategy and how to evaluate the resulting design space and its graphical representation.

After a spate of industrial disasters, the public seeks greater oversight of corporations-so does FDA.

Pfizer Suspends Tanezumab Program; Actavis Appoints CEO; And More.
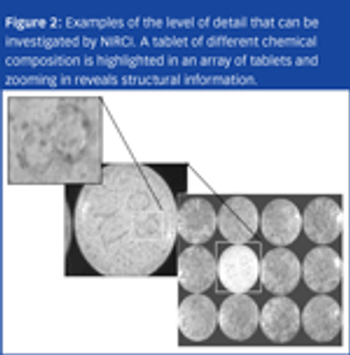
Counterfeit pharmaceuticals are complex products that can vary from their legitimate counterparts both chemically and physically.

Company and People Notes: Valeant and Biovail to Merge; GPhA Names Interim Director; And More.

GSK Acquires Laboratorios Phoenix; Catalent Makes Executive Appointments; And More.

Thermo Fisher to Acquire Fermentas; Pfizer Names Head of R&D; And More.

A timely new book explains techniques for conformational analysis.

Europe moves to place excipient GMP and GDP standards on the same level as active pharmaceutical ingredients.

Too much or too little control can actually lead to the same result.
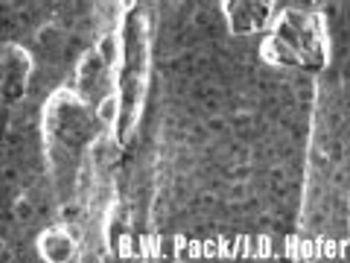
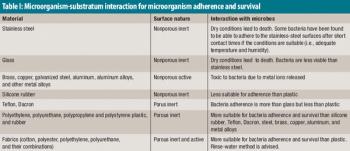
The authors recommend a strategy for classifying similar nonstainless-steel surfaces into three groups based upon the analytical recovery that was observed in this study.

Bayer to Open San Francisco Innovation Hub; Lonza Bioscience Names COO; And More.

Lonza Acquires MODA; Sigma-Aldrich Exec to Retire; And More.