
A roundup of developments on corporate social responsibility from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

A roundup of developments on corporate social responsibility from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

A recently released study by Accenture shows that sustainability is becoming an increasingly important part of the strategies and operations of companies.

Industry's focus on cost cutting has led to a dangerous gap in training and knowledge.

Cases of overlooking proper packaging, reconstitution, directions, and dissolution.

Manufacturers and regulators on both sides of the ocean move to ensure the safety of heparin and other globally distributed drug products.

Industry can meet its responsibility to society by considering innovative pricing and partnerships.

Three therapies under review could help Americans attain a healthy weight.
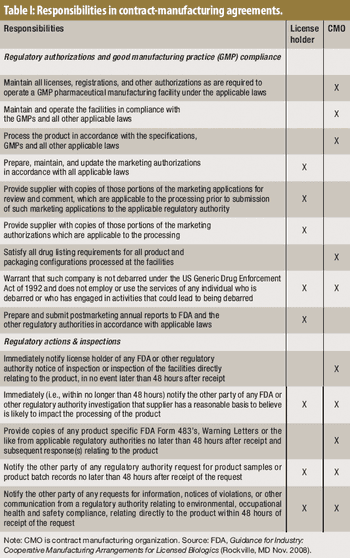
Before embarking on an outsourcing relationship, it's important to be aware of FDA's expectations.

With more hands in the manufacturing pot, a good contingency plan is crucial to success.

The FDA has approved the first generic version of the blockbuster low molecular weight heparin Lovenox (enoxaparin sodium), much to originator sanofi-aventis's displeasure.

Merck and Sinopharm Sign Agreement; Agilent Appoints CFO; And More.

French pharma giant Les Laboratoires Servier SA has been accused of providing "misleading" and "incorrect" information during the EC's antitrust investigation.

In 2009, the European Commission (EC) Customs Union seized 11,462,533 medicines and medical products for suspected violations of intellectual property (IP), according to the Commission's annual report on EU Customs Enforcement of Intellectual Property Rights, which was published last week.

A new report from the International Data Corporation (IDC) shows that life-science companies are increasingly using third-party outsourcing firms to augment or replace their information technology services.

European Medicines Agency Issues Drug Review Update; And More

Researchers claim that a dissolving microneedle patch may be able to offer improved vaccination against influenza compared with traditional needles, and also allow people without medical training to easily and safely administer the vaccine.

Pfizer Ends Second Tanezumab Clinical Program; Catalent VP Joins USP Panel; And More.

ICH Draft Residual Solvents Guideline Published; And More.

The Society of Chemical Manufacturers and Affiliates (SOCMA) expressed "strong concern" over legislation introduced in the US Senate earlier this month regarding chemical-site security.

Merck KGaA (Darmstadt, Germany), a global pharmaceutical and chemical company, completed its acquisition of Millipore (Billerica, MA), a life-science company, last Thursday for an aggregate purchase price of roughly EUR 5.2 billion ($6.7 billion).

The US Food and Drug Administration has joined the Tox21 collaboration, which aims to develop ways to more effectively predict how chemicals will affect the body and environment.

Teva's and Sun Pharmaceuticals' motion to overturn a verdict issued against them in April 2010 for patent infringement has been denied by a US court, leaving the companies at the mercy of pharma giants Pfizer and Nycomed, who have said they will "vigorously" pursue damage claims.

The pharmaceutical industry?s increasing interest in inhaled drugs has prompted several researchers to propose standard dissolution-testing methods for these products.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the July 2010 edition from Gems Sensors and Controls and Telstar.

With the right technology tools, the sponsor organization and the contract manufacturer can have self-service, on-demand, and scheduled access to all manufacturing, quality, and process-development data.

The US Food and Drug Administration announced last week that it will be "conducting a series of inspections in an effort to evaluate industry's compliance and understanding of [21 CFR] Part 11."

Xcelience and Penn Form Joint Venture; Almac Appoints QA Director; And More.

States can reduce their Medicaid programs' healthcare expenditures by changing laws to enable generic drugs to be substituted for branded medications more easily and quickly, according to a new study conducted by CVS Caremark, a large pharmacy healthcare provider.

McNeil Consumer Healthcare, a division of Johnson & Johnson (J&J), has once again expanded the recall of certain OTC products because of a musty or moldy odor, which has been linked to trace amounts of the chemical 2,4,6 triburomoanisole (TBA).

The Laboratory Services Division of the Philippine Food and Drug Administration (FDA) has attained internationally recognized accreditation for its testing and calibration laboratories, according to a July 12, US Pharmacopeia (USP) announcement.