
Udit Batra, current head of Merck KGaA’s life science business, will lead the combined life science businesses following successful completion of Sigma-Aldrich acquisition.

Udit Batra, current head of Merck KGaA’s life science business, will lead the combined life science businesses following successful completion of Sigma-Aldrich acquisition.

As the prevalence of falsified medicines continues to increase, Switzerland is taking measures to secure its supply chain such as the implementation of serialization.

FDA issues a Warning Letter to Hospira S.p.A. for GMP violations at the company’s Liscate, Italy facility.

Baxter voluntarily recalls select lots of IV solutions due to possible particulate matter.

The grants will be offered to investigators conducting research in the field of PCSK9 biology.

Aptar Pharma has partnered with Shanghai Sine Promod Pharmaceutical to develop and launch its new budesonide dry powder inhaler (DPI), which features Aptar Pharma’s user-friendly and cost-effective Twister DPI.

Eppendorf announces that it won the Best New General Lab Product at the Scientists’ Choice Awards.

Companies manufacturing cytotoxic drugs must ensure that staff are given the highest possible levels of protection.

Meggle’s business group, Excipients and Technology, has appointed Alexander Torster, MBA, as its new head of sales and marketing.

Avomeen Analytical Services announces that David W. Riggs, PE will take the role of president of the company.

Cornell Stamoran, Catalent Pharma Solutions vice-president, is elected to the board of trustees of the Pharma & Biopharma Outsourcing Association.

Mylan announces that it would offer Perrigo $29 billion in cash and stock to buy the Irish company.

Theravectys has been granted authorization by the French National Agency for Medicines and Health Product Safety to produce lentiviral vectors for Phase I to Phase III testing at its manufacturing plant.

The move was made to meet an upsurge in Phase I/II GMP campaigns from clients in Europe and the United States.

Novartis will make payments to Juno to settle a patent dispute covering chimeric antigen receptor T-cell (CAR-T) therapies.

The system is compatible with a wide range of industrial CHO cell lines.

Facilities in China, Ireland, Germany, and the United States have been recognized by ISPE in the 2015 Facility of the Year Awards program.

Pall’s acquisition of BioSMB from Tarpon Biosystems expands its downstream continuous processing offerings.

Catalent acquires Pharmapak Technologies, a pharmaceutical packaging company based in New South Wales, Australia.

GlaxoSmithKline announces global vaccines research and design facility to be based in Rockville, MD, USA.
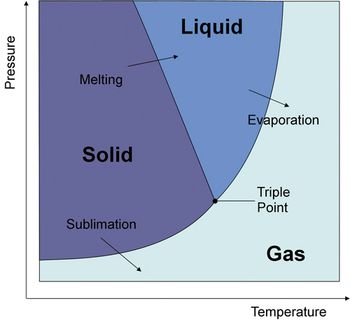
Efficient freeze-drying processes result in time and energy savings, reduced failure rates, and improved batch consistency.

NIH announced positive safety results from the vaccine, VSV-ZEBOV, and found that all patients in the study experienced a strong antibody response.

Drug developers understand the importance of early communication with regulators, but is EMA providing enough flexibility and support to companies?

In this article, industry experts discuss critical analyses for demonstrating biosimilarity.

FDA approves a biosimilar and loses a commissioner in March.

Hope abounds for local drug discovery companies despite challenges at home.

The authors outline a validation approach for the manufacture of a “legacy product,” taking into consideration recent guidelines. The aim is to demonstrate the validity and capacity of the manufacturing process following a change in the manufacturer.

As the prevalence of falsified medicines continues to increase, Switzerland is taking measures to secure its supply chain such as the implementation of serialization.
A review of some of the latest packaging and drug-delivery innovation presented at Pharmapack Europe.

USP announces an implementation date of Jan. 1, 2018 for General Chapters Elemental Impurities-Limits and Elemental Contaminants in Dietary Supplements.