
Merck Serono announces that it will work with Intrexon to develop a cancer therapy using chimeric antigen receptor T-cells.

Merck Serono announces that it will work with Intrexon to develop a cancer therapy using chimeric antigen receptor T-cells.

Rentschler Biotechnologie launches 2000-L single-use bioreactor and announces additional expansion.

Xcellience receives approval to handle both analytical and manufacturing of DEA Scheduled products.

As an affiliate member, TraceLink brings serialization expertise to the Pharma & Biopharma Outsourcing Association.

Drug makers back alternative to FDA labeling update rule.

Horizon Pharma offers to buy Hyperion Therapeutics for $1.1 billion in cash.

Novartis announced that it entered into a multiyear alliance with Aduro Biotech to develop cancer immunotherapies, offering up to $250 million in upfront payments and equity investments.

The agency outlines recommendations for the development and submission of near infrared analytical procedures.

Commissioner Margaret Hamburg discusses globalization challenges and the need for investment in regulatory science during her last weeks at the agency.

Biosimilar applicants will not be required to hand over their biosimilar applications and manufacturing dossiers to innovator companies, determines FDA.

The White House confirmed that it would release a plan to tackle the growing problem of antibiotic-resistant superbugs.

Adroit Science AB will support Recipharm’s pharmaceutical development with solid-state characterization services.

The WHO report outlines possible policies that may help governments reduce the high prices of new medicines.

Germany is a mature market with high healthcare expenditure and pharmaceutical production but future growth could be potentially hindered by cost-saving government policies.

FDA has concluded that Kemwell’s facility, systems, and practices comply with FDA’s requirements and no observations were reported on Form 483.

Bristol-Myers Squibb acquired an exclusive license for Novo Nordisk’s discovery biologics research program.

Genentech plans to invest more than $125 million in an expansion of its fill/finish facility in Hillsboro, OR.

FDA has approved another indication for Eylea, a treatment for diabetic retinopathy in patients with diabetic macular edema.

The commission adopts 24 new and 72 revised texts for inclusion in the European Pharmacopoeia.

Emergent BioSolutions announced FDA approval of Anthrasil, an inhalable treatment that targets Anthrax toxins, as well as a contract with BARDA to develop NuThrax, an anthrax vaccine candidate.

The event will take place at the Jakarta International Expo, Indonesia from April 8–10, 2015.

The agency provides guidance on determining the need for environmental assessments for gene therapies, vectored vaccines, and viral and microbial products.

Clinical trial results suggest that monoclonal antibodies targeting the function of proinflammatory cytokine IL-17A in psoriasis may be significantly superior to other treatments.

Guidance provides labeling standards and an implementation guide for electronic submission of lot distribution reports.

The company’s new corporate name will be simply Biogen.

Recipharm expects to be rolling out further serialization projects during 2015.

The investments made in analytical and process development equipment will enable Laurus Synthesis to offer chemistry services to its clients.

International day for fighting infection sees European infection specialists advocating vaccines as a viable solution to tackle antimicrobial resistance.

UPS expands its special commodities program to more countries.
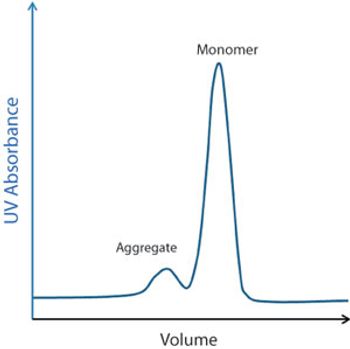
Several chromatographic resins are available for downstream purification.