
Data governance is necessary for compliance with current regulatory expectations for data integrity in pharmaceutical R&D and manufacturing organizations.

Data governance is necessary for compliance with current regulatory expectations for data integrity in pharmaceutical R&D and manufacturing organizations.

Ralph Gosden, head of product development at Catalent’s Swindon, United Kingdom site, explains the opportunities of orally disintegrating tablets as a patient-centric dosage form.

Experts from Colorcon share insights on how manufacturers can play a role in minimizing the risk of medication errors by ensuring that the medicines they develop are well differentiated, especially between dosages of the same product.

Pharmaceutical companies are increasingly adopting patient-centric formulation development to ensure that drug products adequately meet the needs of end-users in all target patient populations.

The shift toward continuous manufacturing among European pharmaceutical manufacturers has not been accompanied by a similar strong increase in the use of automation and sensor-based on-line monitoring.

The level of formality in change control may be holding back your SOP progress, according to Siegfried Schmitt, principal consultant at PAREXEL.
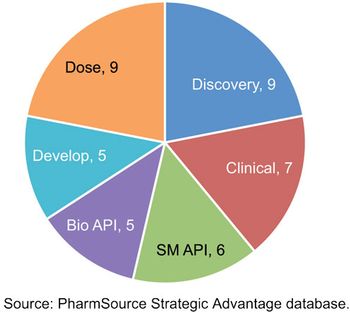
Recent acquisitions are creating CDMOs with scale that rivals global bio/pharma.

The revised Akers-Agalloco aseptic risk assessment and mitigation method includes two sub-methods that can be used independently for risk assessment.

FDA seeks to focus on problematic facilities and inform firms quickly about site problems.

Industry experts were honored for business, scientific, and social contributions.

Taste masking increases drug acceptability and medication adherence in pediatric, geriatric, and other special patient populations.

Creating a quality culture can prevent the costs and challenges associated with receiving a consent decree.
In Part I of this article series, the regression control chart method for identifying out-of-trend data in pharmaceutical stability studies is investigated, and an improved approach is suggested.

The 300-gallon process vessel from Ross Charles can be customized according to specific user needs.

The K3-PH-ML-D4-QT35 feeder from Coperion K-Tron is suited for multi-feeder clustering in a variety of continuous processes.
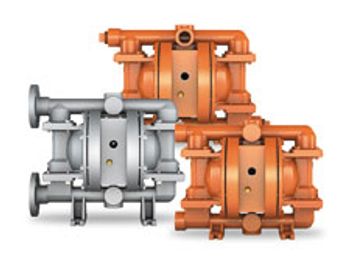
Wilden's air-operated, double-diaphragm pumps feature bolted product containment, self-priming and dry-run capabilities, and are direct replacements for existing clamped and bolted pumps from the company.
Model-based formulation and technology selection methodologies facilitate rapid product development.

Inadequate endotoxin testing places patients at risk. Knowing the relative strengths and weaknesses of available test methods is crucial to maintaining quality and safety.

Click the title above to open the Pharmaceutical Technology November 2017 issue in an interactive PDF format.

The Almac Pod, a temperature-controlled shipping solution service for biologics and other temperature-sensitive products, is compliant with good distribution practices and comes in three models.

Despite GxP and data-management challenges, pharma is moving toward new models for clinical trial logistics.