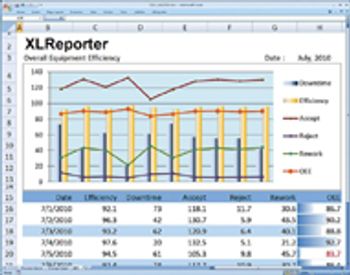
Editors' picks of pharmaceutical science and technology innovations.

Editors' picks of pharmaceutical science and technology innovations.

Abuse-deterrent combination drugs represent a niche area in formulation development.

USP is working to ensure quality standards and to increase public information.

A recent book offers an excellent overview of cleaning validation.

IP rights and levels of innovation have opened a bit of controversy regarding decisions being made by Indian courts and legislators.

An analysis of the approaches and tools used to tackle the problem of poorly soluble drugs.

Industrial and academic partnerships forge new territory in solid-state chemistry.

A conversation with Mike de la Montaigne, president of Eisai Machinery, USA Inc., about the possibilities for conducting fully automated product inspections.

As technology advances, industry's needs are growing.

Scientists and practitioners must work together for the overall good of the patient.

A new center may provide evidence for improving care, but could discourage coverage of treatments.
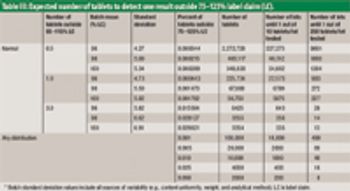
The authors describe a modified version of the Large-N test used to determine content uniformity.

A Q&A with officers of the departments of State Food and Drug Administration, China, moderated by Ji Xie.
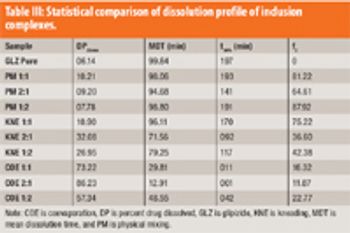
The authors describe the development of an inclusion complex of GLZ and formulated an extended-release dosage form based on osmotic technology.

Drug manufacturers have to be more than just "audit ready."

Remaining calm, cool, and collected during mergers and inspections is a feat in itself.
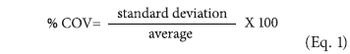
The author examines the process of method development, with reference to ISO 13320:2009 and relevant monographs from the United States and European pharmacopoeias.
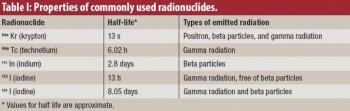
The authors discuss gamma scintigraphy as a technique for in vivo evaluation of drugs and delivery systems.

The authors demonstrated that ODTs can be obtained by direct compression of a mixture of starch, fructose, and SMCC.

Merck and AstraZeneca Tackle Cervical Cancer and Tuberculosis.

Post recession and beyond, which contract service providers will still be standing?

Connecting science and policy might increase support for innovation.

Used packaging equipment can be a cost-saving, time-saving alternative to new machines.

The authors present an update to the Wyeth/BASF experience with the IPEC Novel Excipient Safety Evaluation Procedure.

Stuart Needleman, president of active-ingredient development and manufacturing at Aptuit, discusses industry trends and challenges.

Applied statisticians are forever searching for the enemy of quality-variability.

Solubilizing the Insoluble