
Robots Lend a Hand

Robots Lend a Hand

Brief pharmaceutical news items for September 2009.

Oostdyk discusses the latest industry developments and trends.

Thanks to their keen observations, these auditors reveal the true culprits of deviations.

After years of promomting QbD concepts, FDA's ready to take action on nonconformers.

Robotic systems provide flexibility and efficiency (and they're not as difficult to use as you think). This article contains bonus online-exclusive material.

An outstanding new book reviews alternative solvents with an eye to sustainable pharmaceutical processes.

Contract-service providers are expanding their offerings in this slow-growth environment.

A second-generation and green manufacturing process for testosterone provided economic and ecological benefits.

Editors' Picks of Pharmaceutical Science & Technology Innovations

Authenticating tools help identify counterfeit drug products. This article contains bonus online-exclusive material.
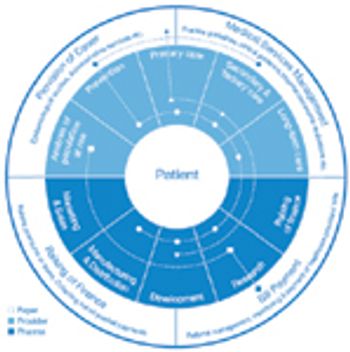
Personalized medicine and integrated healthcare delivery require new business and pricing models. This article contains bonus online-exclusive material.

Select contract manufacturing organizations roll out expansions for production of active pharmaceutical ingredients and intermediates.

Health crises generate support for new vaccines and treatments for diseases found in developing nations.

Representatives of Japan's MHLW report on recent ICH activities and what the ministry expects from Q11.

Pfizer uses green-chemistry in a second-generation manufacturing route for gabapentin.
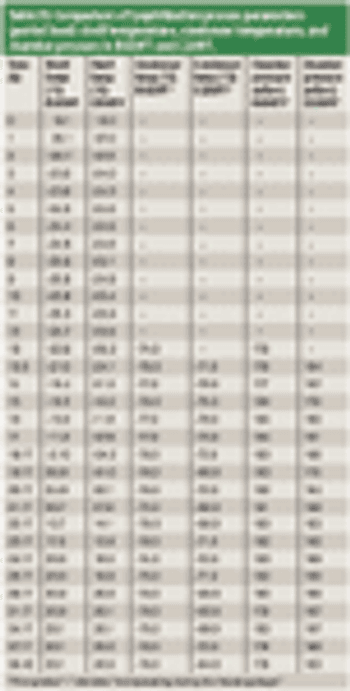
The authors describe a comprehensive methodology for establishing functional equivalence among various lyophilizers.

As new process validation guidelines emerge, industry needs to reinvent how it releases product.

IPEC's new stability testing guide takes into account the full supply chain's storage conditions.
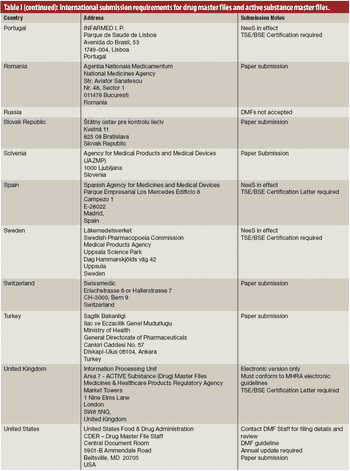
The author describes several issues in creating drug master files and active substance files for active pharmaceutical ingredients and intermediates.

Designated as a "pharmerging market," Brazil is revamping its pricing models.