
Identify the warning signs and follow best practices for refurbishment to improve tablet press yields.

Identify the warning signs and follow best practices for refurbishment to improve tablet press yields.

New technologies in the digital factory enhance quality, efficiency, and flexibility for bio/pharmaceutical manufacturing.

Do patients get what they pay for when they demand cheaper drugs?

Understanding the API, delivery mechanism, and excipient functionality is essential to solving drug solubility challenges.

A monoplant may offer greater supply security and flexibility for specialist medicines.

Bio/pharma companies facing new challenges in light of the increasing HPAPI market may benefit from outsourcing.

As biologics continue to push boundaries, the industry needs to take a holistic approach to formulation to ensure success.

As drug pricing comes under the microscope internationally, it appears that collaboration with all stakeholders is key to tackling the issue.

The European generics and biosimilars sector is working on the creation of a single pathway to accelerate development of and access to medicines.
Harmonization of best practices and regulatory requirements will enable developers to find the best stability testing approach.

Containment valves and smart monitoring can keep employees safe and improve manufacturing efficiency when handling potent APIs, intermediates, and solid-dosage drugs.

Simplification is crucial to maintain data integrity, according to Siegfried Schmitt, PhD, vice-president, technical at PAREXEL Consulting.

FDA expects more than 200 investigational new drug applications for cell and gene therapies by 2020, causing the agency to strengthen its regulatory program.
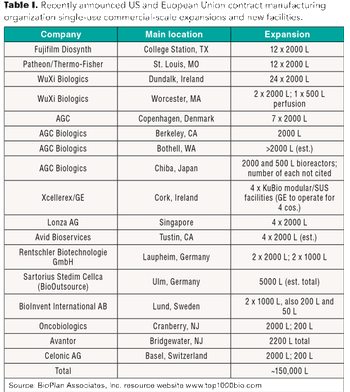
Single-use systems can be a cost savings for CMOs, and these savings can be passed on to clients and, ultimately, to patients.

While allogeneic therapies can use existing regulatory and quality frameworks, autologous treatments will require pharma’s adoption of true just-in-time and right-first-time concepts, says consultant James Blackwell.

Optical coherence tomography can improve quality control and development of coated dosage forms by allowing film thickness to be measured in real time.

The DrySyn UNO Base by Asynt offers a way to conduct safe experiments in academic teaching and research labs.

The Pulse-Flow PTA dense phase pneumatic conveying system from Gericke USA directs air or nitrogen into the pressure vessel and pipeline in timed, regular pulses that form the dry material into separate, wavelike, pulsed slugs.

UniFlow FM Fume Hoods by HEMCO are suited for synthesis, distillation, and other rack-type operations where a tall apparatus is used or equipment is rolled into the work area.

The VMC VersaMix by Charles Ross & Son Company is suited for temperature-critical formulations of medium to high viscosity under stringent vacuum/pressure constraints.

Click the title above to open the Pharmaceutical Technology March 2019 issue in an interactive PDF format.