
Pharmaceutical Technology Europe
The attraction of nasal administered therapeutic agents is obvious, including faster onset of action, increased compliance and avoiding degradation during first pass metabolism.

Pharmaceutical Technology Europe
The attraction of nasal administered therapeutic agents is obvious, including faster onset of action, increased compliance and avoiding degradation during first pass metabolism.

Pharmaceutical Technology Europe
Particle shape is an important parameter to monitor in the pharmaceutical sector, but has, historically, been too complicated to measure and be utilized on a routine basis. A newly developed digital technique could change this.

Pharmaceutical Technology Europe
With the prospect of a possible pandemic the Swiss company, Roche, has found sales of its flu drug have rapidly increased but its patent is being questioned. Roche's Tamiflu sales have inflated by 263% in the first 9 months of this year but discussions of patent suspension from several countries and United Nations Secretary General Kofi Annan is pressurizing the company.

Pharmaceutical Technology Europe
The introduction of new measures on advertising and promotional materials for prescription medicines could delay UK launches. The Medicines and Healthcare products Regulatory Agency (MHRA) has admitted that the checks, which have been introduced in response to a Commons' Health Select Committee report, could extend a launch by at least 2 weeks.
Pharmaceutical Technology Europe
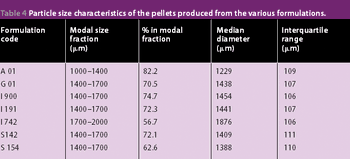
The previous studies on the incorporation of glyceryl monostearate into pellets by extrusion/spheronization has been extended to include a range of grades of this material plus a mixed medium chain partial glyceride and two glycerol esters of hydrogenated natural glycerides as described in this article.
Pharmaceutical Technology Europe
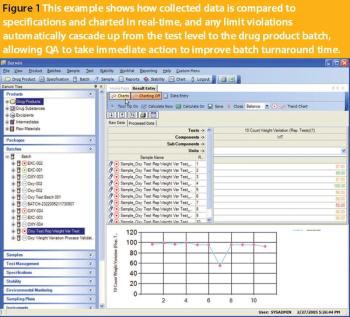
To produce an application or solution for a specific domain, a vendor must demonstrate a thorough understanding of the domain and its specific challenges.

Pharmaceutical Technology Europe
In applying a visually clean standard, any residue related to the cleaning process that is visible on the surface should constitute a failure.
Pharmaceutical Technology Europe
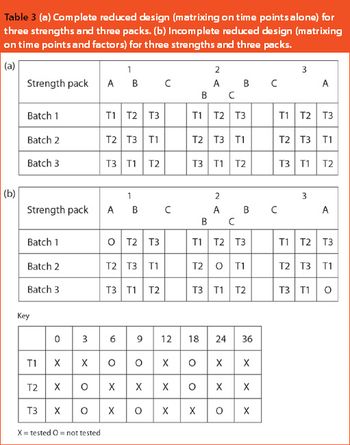
This article looks at the use of bracketing and matrixing to lower the number of stability samples required and, consequently, reduce the cost of sample production, testing and management. There is a common misconception that regulatory authorities will not accept such methods, but there is actually an International Conference on Harmonization guideline (ICH Q1D) on the subject. In fact, many of these designs have already been accepted and FDA members were among the first to describe matrixing.

Pharmaceutical Technology Europe
The monopoly held by the large pharmaceutical companies within drug discovery could be falling into the hands of biotechnology firms claims Frost and Sullivan. The consultancy company's report, Biochips Technology Redefines Process of Drug Discovery, states that biochip manufacturers are encouraging end users to accept the new technology by providing novel and effective solutions.
Pharmaceutical Technology Europe
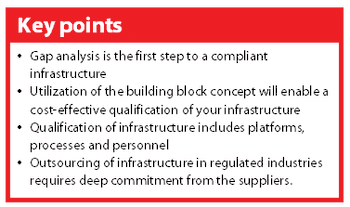
A new Good Automated Manufacturing Practice (GAMP) guide on IT Infrastructure Control & Compliance was launched in Chicago (IL, USA) 23 August 2005.1 The guide is intended to support pharmaceutical companies in their effort to establish a well-defined and compliant infrastructure. This article discusses different aspects of the guide that may support your organization in getting — and keeping — your infrastructure under control.

Pharmaceutical Technology Europe
Exploring a simple and systematic approach to validating an FMS.