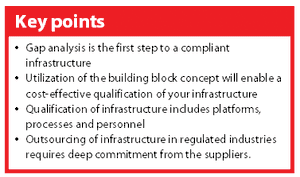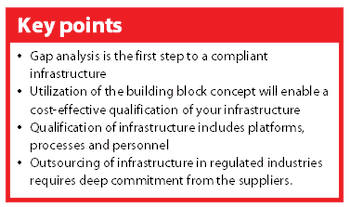
A new Good Automated Manufacturing Practice (GAMP) guide on IT Infrastructure Control & Compliance was launched in Chicago (IL, USA) 23 August 2005.1 The guide is intended to support pharmaceutical companies in their effort to establish a well-defined and compliant infrastructure. This article discusses different aspects of the guide that may support your organization in getting — and keeping — your infrastructure under control.