
Pharmaceutical Technology Europe
A possible recession in the US and a global credit crunch are going to be major factors in the amount of money available as shareholders tighten their purse-strings.

Pharmaceutical Technology Europe
A possible recession in the US and a global credit crunch are going to be major factors in the amount of money available as shareholders tighten their purse-strings.

Pharmaceutical Technology Europe
As globalization of drug development and manufacturing gathered steam in the early part of this decade, many pharmaceutical companies in the newly-favoured regions of Asia, Eastern Europe and Latin America added contract services as adjuncts to their generic API and dosage form businesses. These new units, which in India are known under the unfortunate acronym of CRAMS (contract research and manufacturing services), were opportunistic responses to the growing outsourcing trends. Unfortunately, in many cases, they were not truly strategic commitments to the services business.
Pharmaceutical Technology Europe
Pharmaceutical Technology Europe
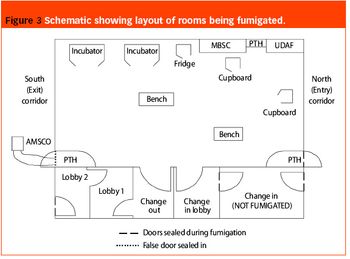
As part of a major project to design and build a new bulk vaccine antigen plant, the authors were asked to investigate and implement a suitable fumigation system for clean room decontamination. The facility was designed to handle and contain live influenza virus, and has clean room suites designed to containment levels CL2 and CL3 according to the Approved Code Of Practice and Guidance (ACOP, Control of Substances Hazardous to Health 4th Edition). From the outset, specific areas within the facility were identified as requiring fumigation and this formed part of the initial design brief.

Pharmaceutical Technology Europe
Deciding where in the world to locate a new plant is a key decision for any pharma or biotech company - and there has never been more choice. Europe and the US now compete with the Far East and India, and what about the new EU states? Might Lithuania or Estonia turn out to offer advantages compared with France or Germany when it comes to finding the best place to take a new drug forward to the market place?

Pharmaceutical Technology Europe
Robotization of end-of-line packaging systems enables manufacturers to maintain high production rates while accommodating flexible and varied product packaging requirements.

Pharmaceutical Technology Europe
We are currently experiencing a problem with one of our tablet lines. While the tablets appear white immediately after manufacture, after a time many of the tablets begin to take on a yellowish appearance. Could this be an issue that surface analysis could help resolve?