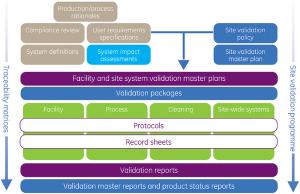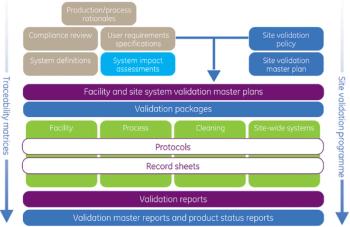
Despite the fact that regulatory compliance is fundamental to the pharmaceutical industry, too many manufacturers lose millions of euro in revenue because of poorly or incorrectly validated facilities. With increasingly demanding global regulations and guidance, rising manufacturing costs and dwindling product portfolios, it is vital to achieve efficient and effective compliance of facilities, processes and equipment to retain market competitiveness.