
Washington, DC (Apr. 5)-The Pharmaceutical Research and Manufacturers of America joined the debate on follow-on biologics when senior vice-president Caroline Loew issued a statement recommending caution.

Washington, DC (Apr. 5)-The Pharmaceutical Research and Manufacturers of America joined the debate on follow-on biologics when senior vice-president Caroline Loew issued a statement recommending caution.


Brussels, Belgium (Mar. 22)-The European Commission?s (EC) Directorate-General for Enterprise and Industry (Brussels, Belgium) is asking manufacturers, distributors, and users of human-pharmaceutical excipients to participate in an online questionnaire on the effect of various policy options. Responses will be used to prepare a directive on good manufacturing practices (GMPs) for certain excipients.

Rockville, MD (Mar. 1)-The number of US Food and Drug Administration inspections of biologic and drug-manufacturing facilities declined to 4237 in fiscal 2006, according to a report by the Office of Regulatory Affairs.

BIO Raises Concerns About Studies on Follow-On Biologics; Green Chemistry Reduces Costs and Waste; Novel Polymer–DNA Delivery System; WHO Stresses Lack of Capacity for Pandemic Flu Vaccine; EGA Concerned About Regulatory Workload in Approving Generics

Indian pharmaceutical machine manufacturers (IPMMs) are exceptional among their foreign counterparts. Historically similar to the Chinese with regard to copycat practices, patent infringements, and substandard quality, the IPMMs have made great strides in innovation and collaboration to break free from the shackles of this paradigm.

Agawam, MA (Mar. 26)-Contract manufacturing and testing laboratory Microtest signed a manufacturing deal with Antisoma to produce AS1411, a new drug being developed for the treatment of various cancers.

South San Francisco, CA (Mar. 28)-Genentech, Inc. announced plans to invest $140 million in 1000-liter manufacturing facility in Singapore.

Washington, DC (Mar. 26)-The congressional Committee on Oversight and Government Reform held a hearing to evaluate the cost of biotech drugs as well as strategies for establishing an approval process for the US Food and Drug Administration approval of generic versions of these drugs.
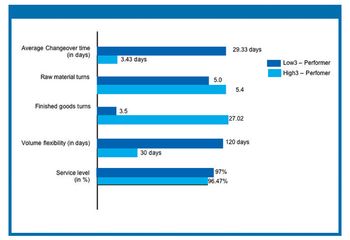
Improving performance at an active pharmaceutical ingredient manufacturing plant involves an integrated approach that incorporates methods for optimizing total production management, quality control and assurance, and inventory management. The authors analyze results from a recent benchmarking study to evaluate the critical success factors in high-performing API manufacturing plants.

Rockville, MD (Mar. 23)-The US Food and Drug Administration has submitted to Congress its final proposals for reauthorizing the fourth Prescription Drug User Fee Act (PDUFA IV), which will follow the expiration of the current user-fee on September 30, 2007.


Berlin and Basel, Switzerland (Mar. 26)-Bayer Schering Pharma AG and Novartis reached an agreement over multiple sclerosis therapy ?Betaseron.?


Paris (Mar. 15)-Sanofi-Aventis plans to close its manufacturing site in Waterford, Ireland.

Buffalo, NY (Mar. 7)-Scientists at the University at Buffalo?s Institute for Lasers, Phtonics, and Biophotonics and Roswell Park Cancer Institute have developed a drug delivery system comprising 100-nm nanocrystals of pure HPPH, (2-devinyl-2-1'-hexyloxyethyl pyropheophorbide).


Vancouver, BC, Canada-Researchers from the University of British Columbia are developing a new method for delivering Amphotericin B, a potent antifungal agent for fatal blood-borne fungal infections that currently must be administered intravenously.

Berlin, Germany (Mar. 2)-Estimating it would save approximately EUR 700 million ($917.7 million), Bayer HealthCare will integrate the activities of its Pharma division with those of the former Schering AG, Germany.

Affectis, Dalton, Celgene, Novartis, GSK, More

Rockville, MD (Mar. 1)-Sending Warning Letters to 8 manufacturers and 12 distributors, The US Food and Drug Administration ordered a stop to the manufacture and distribution of unapproved drug products containing ergotamine tartrate.

The proposed rule would impose new record-keeping requirements on some 1200 manufacturing establishments.


Enschede, Netherlands (Feb. 26)-Scientists from the University of Twente?s MESA+ Institute for Nanotechnology have designed a novel delivery system by combining synthetic iron-containing polymers with DNA macromolecules.

Rockville, MD (Jan. 5)-The US Food and Drug Administration issued a Warning Letter to Bell-More Laboratories following the agency?s August 2006 inspection of the company?s Hampstead pharmaceutical facility.

Rockville, MD (Feb. 1)-The US Food and Drug Administration issued a revised warning letter to Actavis Totowa, LLC, citing ?significant deviations from the current Good Manufacturing Practice regulations.?

Aptuit, AstraZeneca, Corautus, FDA, more

Geneva, Switzerland (Feb. 16)-During a two-day meeting, the World Health Organzation announced ?encouraging progress? in the development of a pandemic influenza vaccine but admitted that the industry still ?lacks the manufacturing capacity to meet potential pandemic influenza vaccine demand.?


Washington, DC (Feb. 14)?A congressional team has reintroduced the ?Access to Life-Saving Medicine Act,? which establishes a process through which the US Food and Drug Administration can approve generic copies of biologic drugs.